Prolonged Thermal Relaxation of the Thermosetting Polymers
Abstract
:1. Introduction
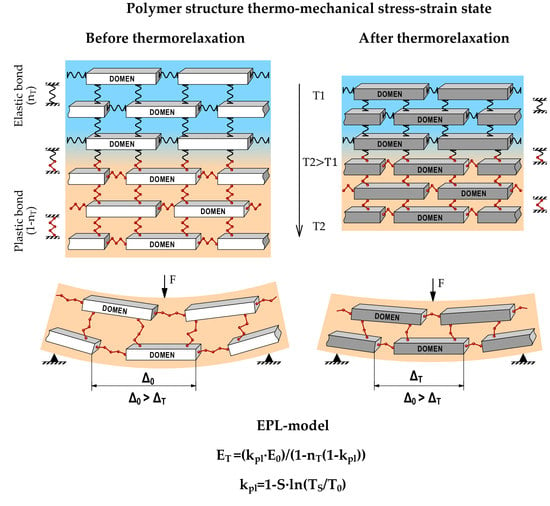
- Molecular inter-domain bonds determine the rheology of ITZ and make its character viscose or viscoelastic [16,17,18,19,20,21]. It reflects on the viscoelastic deformative properties of the overall polymer structure. ITZ is an inter-domain surface and matrix of the glassed conglomerate. Due to its matrix character, the ITZ is endless and can mass remove and create sliding surfaces for deformation under load and heating.
- Thermo-gravimetry and modulus of elasticity after heating of glassed polymers.
- DMA research for glassing onset temperature points before and after thermo-relaxation.
- Study of the polymer “deformation modulus-temperature” dependence before and after thermo-relaxation.
2. Materials and Methods
2.1. Materials
- Epoxy resin KER 828, with the following main characteristics: Epoxy Group Content (EGC) 5308 mmol/kg, Epoxide Equivalent Weight (EEW) 188.5 g/eq, viscosity at 25 °C 12.7 Pa.s, HCl 116 mg/kg, and total chlorine 1011 mg/kg. Manufacturer: KUMHO P&B Chemicals, Gwangju, South Korea.
- Hardener for epoxy resin methyl tetrahydrophthalic anhydride with the following main characteristics: viscosity at 25 °C 63 Pa.s, anhydride content 42.4%, volatile fraction content 0.55%, and free acid 0.1%. Manufacturer: ASAMBLY Chemicals company Ltd., Nanjing, China.
- Alkofen (epoxy resin curing accelerator) with the following main characteristics: viscosity at 25 °C 150 Pa.s, molecular formula C15H27N3O, molecular weight 265, and amine value 600 mg KOH/g. Manufacturer: Epital JSC, Moscow, Russian Federation.
- Resol phenolic resin SFRZ-309 with the following main characteristics: viscosity at 25 °C 700 mPa.s, not more than 20% (m/m) water, and not more than 20% (m/m) free phenol. Manufacturer: FCP “Sverdlov Plant”, Dzerzhinsk, Russian Federation.
2.2. Methods
3. Results and Discussion
3.1. Polymer Mass Lost under Heating and Modulus of Elasticity after Heating Research
3.2. Polymers before and after Thermo-Relaxation DMA
3.3. Testing the Polymer Elasticity Modulus concerning Temperature and Entropy Factor after Thermo-Relaxation
4. Conclusions
- Volatile fractions’ mass loss in the range of 5–10%.
- The glassing onset point increased by 1.3–1.7 times.
- The normal temperature modulus of elasticity in bending after prolonged exposure at elevated temperatures did not change significantly. In contrast, the moduli of elasticity at high temperatures for all types of binders increased by several times.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slutsker, A.I.; Sanphirova, T.P.; Yastrebinskii, A.A.; Kuksenko, V.S. Structure and reversible deformability of oriented crystallizing polymers. J. Polym. Sci. Part C Polym. Symp. 1967, 16, 4093–4101. [Google Scholar] [CrossRef]
- Flory, P.J.; Erman, B. Theory of elasticity of polymer networks. 3. Macromolecules 1982, 15, 800–806. [Google Scholar] [CrossRef]
- Dill, K.A.; Bromberg, S.; Stigter, D. Polymer Elasticity & Collapse. In Molecular Driving Forces; Garland Science: New York, NY, USA, 2018; pp. 659–684. [Google Scholar] [CrossRef]
- Lomakin, V.A.; Ogibalov, P.M.; Teters, G.A. Problems of the theory of deformation of polymeric materials. Polym. Mech. 1972, 8, 377–385. [Google Scholar] [CrossRef]
- Vashisth, A.; Ashraf, C.; Bakis, C.E.; van Duin, A.C.T. Effect of chemical structure on thermo-mechanical properties of epoxy polymers: Comparison of accelerated ReaxFF simulations and experiments. Polymer 2018, 158, 354–363. [Google Scholar] [CrossRef]
- Chan, C.H.; Chia, C.H.; Thomas, S. Physical Chemistry of Macromolecules: Macro to Nanoscales; Apple Academic Press: Palm Bay, FL, USA, 2014. [Google Scholar]
- Kuperman, A.M.; Turusov, R.A.; Gorenberg, A.Y. Study of elastic and strength properties of hybrid and gradient polymer composites. Compos. Mech. Comput. Appl. 2010, 1, 361–373. [Google Scholar] [CrossRef]
- Rousseau, I.A.; Xie, T. Shape memory epoxy: Composition, structure, properties and shape memory performances. J. Mater. Chem. 2010, 20, 3431–3441. [Google Scholar] [CrossRef]
- Korolev, A.; Mishnev, M.; Zherebtsov, D.; Vatin, N.I.; Karelina, M. Polymers under Load and Heating Deformability: Modelling and Predicting. Polymers 2021, 13, 428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hankett, J.; Chen, Z. Molecular-level Understanding of Adhesion Mechanisms at the Epoxy/Polymer Interphases. ACS Appl. Mater. Interphases 2012, 4, 3730–3737. [Google Scholar] [CrossRef]
- Kuksenko, V.S.; Ovchinnikov, V.A.; Slutsker, A.I. Elasticity of the intercrystallite zones and the mechanical properties of oriented polymers. Polym. Mech. 1969, 5, 891–895. [Google Scholar] [CrossRef]
- Miller, R.; Ferri, J.K.; Javadi, A.; Krägel, J.; Mucic, N.; Wüstneck, R. Rheology of interfacial layers. Colloid Polym. Sci. 2010, 288, 937–950. [Google Scholar] [CrossRef]
- Solar, M.; Qin, Z.; Buehler, M.J. Molecular mechanics and performance of crosslinked amorphous polymer adhesives. J. Mater. Res. 2014, 29, 1077–1085. [Google Scholar] [CrossRef]
- Arzhakov, M. Relaxation in Physical and Mechanical Behavior of Polymers, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Green, A.E.; Naghdi, P.M. Thermoelasticity without energy dissipation. J. Elast. 1993, 31, 189–208. [Google Scholar] [CrossRef]
- Jensen, M.; Jakobsen, J. Effect of cure cycle on enthalpy relaxation and post shrinkage in neat epoxy and epoxy composites. J. Non-Cryst. Solids 2016, 452, 109–113. [Google Scholar] [CrossRef]
- Rouxhet, P.G.; Doren, A.; Dewez, J.L.; Heuschling, O. Chemical composition and physico-chemical properties of polymer surfaces. Prog. Org. Coat. 1993, 22, 327–344. [Google Scholar] [CrossRef]
- Lodge, T.P.; Muthukumar, M. Physical Chemistry of Polymers: Entropy, Interactions, and Dynamics. J. Phys. Chem. 1996, 100, 13275–13292. [Google Scholar] [CrossRef]
- Pepicelli, M.; Jaensson, N.; Tregouët, C.; Schroyen, B.; Alicke, A.; Tervoort, T.; Monteux, C.; Vermant, J. Surface viscoelasticity in model polymer multilayers: From planar interfaces to rising bubbles. J. Rheol. 2019, 63, 815–828. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Wang, X.; Xing, Z.; Fu, Y.Q. A cooperative domain model for multiple phase transitions and complex conformational relaxations in polymers with shape memory effect. J. Phys. D Appl. Phys. 2019, 52, 1361–6463. [Google Scholar] [CrossRef]
- Johnsen, J.; Clausen, A.H.; Grytten, F.; Benallal, A.; Hopperstad, O.S. A thermo-elasto-viscoplastic constitutive model for polymers. J. Mech. Phys. Solids 2019, 124, 681–701. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L. Mechanisms of the complex thermo-mechanical behavior of polymer glass across a wide range of temperature variations. Polymers 2018, 10, 1153. [Google Scholar] [CrossRef] [Green Version]
- Shaw, M.T.; MacKnight, W.J. Introduction to Polymer Viscoelasticity, 3rd ed.; Wiley Blackwell: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Sogolova, T.I.; Demina, M.I. Temperature dependence of the mechanical properties of polymers of different chemical structure in the temperature range from 4.2 to 300 °K. Polym. Mech. 1977, 13, 333–337. [Google Scholar] [CrossRef]
- Moroz, J.D.; Nelson, P. Entropic elasticity of twist-storing polymers. Macromolecules 1998, 31, 6333–6347. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wen, H.; Wu, Y. Computational Thermomechanical Properties of Silica-Epoxy Nanocomposites by Molecular Dynamic Simulation. Polymers 2017, 9, 430. [Google Scholar] [CrossRef]
- Gol’dman, A.Y.; Murzakhanov, G.K.; Soshina, O.A. Temperature-time analogy for thermorheologically complex polymeric materials. 1. Mixtures of partially crystalline polymers with elastomers. Polym. Mech. 1977, 13, 516–522. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.; Fei, F.; Wang, Y.; Liu, Y.; Leng, J. Modeling mechanical behavior of epoxy-shape memory polymers. In Behavior and Mechanics of Multifunctional Materials and Composites, Proceedings of the SPIE, San Diego, CA, USA, 10–14 March 2013; International Society for Optics and Photonics: Bellingham, WA, USA, 2013. [Google Scholar] [CrossRef]
- Khan, A.S.; Lopez-Pamies, O.; Kazmi, R. Thermo-mechanical large deformation response and constitutive modeling of viscoelastic polymers over a wide range of strain rates and temperatures. Int. J. Plast. 2006, 22, 581–601. [Google Scholar] [CrossRef]
- Cheng, H.L.; Wang, J.; Huang, Z.P. A thermo-viscoelastic constitutive model for compressible amorphous polymers. Mech. Time-Depend. Mater. 2010, 14, 261–275. [Google Scholar] [CrossRef]
- Lexcellent, C.; Butaud, P.; Foltête, E.; Ouisse, M. A Review of Shape Memory Polymers Thermomechanical Modelling: Analysis in the Frequency Domain. In Advances in Shape Memory Materials; Sun, Q., Matsui, R., Takeda, K., Pieczyska, E., Eds.; Springer: Berlin, Germany, 2017; Volume 73, pp. 57–80. [Google Scholar] [CrossRef]
- Shenogina, N.B.; Tsige, M.; Patnaik, S.S.; Mukhopadhyay, S.M. Molecular Modeling Approach to Prediction of Thermo-Mechanical Behavior of Thermoset Polymer Networks. Macromolecules 2012, 45, 5307–5315. [Google Scholar] [CrossRef]
- Lu, H.; Huang, W.M. On the origin of the Vogel-Fulcher-Tammann law in the thermo-responsive shape memory effect of amorphous polymers. Smart Mater. Struct. 2013, 22, 105021. [Google Scholar] [CrossRef]
- Lu, H.; Leng, J.; Du, S. A Phenomenological Approach for the Chemo-Responsive Shape Memory Effect in Amorphous Polymers. Soft Matter 2013, 9, 3851–3858. [Google Scholar] [CrossRef]
- Russian State Standard GOST 25.604-82 Design Calculation and Strength Testings. Methods of Mechanical Testing of Polymeric Composite Materials. Test for Bending Properties at Normal, Elevated, and Low Temperatures. Available online: https://docs.cntd.ru/document/1200012862 (accessed on 25 November 2021).
- Horta Muoz, S.; Serna Moreno, M.C. Multiaxial stress and strain analysis on laminated plates under different flexural loading rates. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Roskilde, Denmark, 7–10 September 2020. [Google Scholar] [CrossRef]
- Serna Moreno, M.C.; Romero Gutiérrez, A.; Martínez Vicente, J.L. Different response under tension and compression of unidirectional carbon fibre laminates in a three-point bending test. Compos. Struct. 2016, 136, 706–711. [Google Scholar] [CrossRef]










| No. | Compound | Designation | Thermogravimetric Analysis | Three-Point Bending Test at Elevated Temperature |
|---|---|---|---|---|
| 1 | Epoxy resin (Ker 828 52.5% + MTHPA 44.5% + alkofen 3%) | EP | + | + |
| 2 | Phenolic resin (SFZ-309) 100% | PF | + | + |
| 3 | Epoxy-phenolic resin (KER 828 45% + SFZ-309 55%) | EP-PF | + | + |
| No. | Compound | Width, mm | Thickness, mm |
|---|---|---|---|
| 1 | Epoxy resin (Ker 828 52.5% + MTHPA 44.5% + alkofen 3%) | 8.72 | 3.48 |
| 2 | Phenolic resin (SFZ-309) 100% | 5.95 | 2.8 |
| 3 | Epoxy-phenolic resin (KER 828 45% + SFZ-309 55%) | 7.03 | 2.95 |
| Compound | Temperature, °C | Efact, MPa | S, J/J | kpl | Ecalc, MPa | % Derivation |
|---|---|---|---|---|---|---|
| Epoxy before thermo- relaxation | 25 | 3028 | 3.6 | 1.000 | - | - |
| 70 | 2567 | 0.494 | 2450 | −4.6 | ||
| 80 | 2360 | 0.390 | 2227 | −5.6 | ||
| 90 | 1868 | 0.290 | 1899 | 1.7 | ||
| 110 | 893 | 0.097 | 960 | 7.5 | ||
| Epoxy after thermo- relaxation | 25 | 2950 | 2.8 | 1.000 | - | |
| 70 | 2650 | 0.606 | 2610 | −1.5 | ||
| 80 | 2600 | 0.523 | 2443 | −6.0 | ||
| 90 | 2365 | 0.448 | 2259 | −4.4 | ||
| 110 | 1870 | 0.297 | 1878 | 0.4 | ||
| 115 | 1700 | 0.261 | 1817 | 6.9 | ||
| 120 | 1000 | 0.225 | 1618 | 61.8 | ||
| 130 | 724 | 0.154 | 1307 | 80 | ||
| 140 | 338 | 0.086 | 858 | 153 | ||
| 150 | 208 | 0.019 | 231 | 6.3 |
| Compound | Temperature, °C | Efact, MPa | S, J/J | kpl | Ecalc, MPa | % Derivation |
|---|---|---|---|---|---|---|
| Phenolic before thermo-relaxation | 25 | 2935 | 2.5 | 1 | - | - |
| 110 | 1823 | 0.372 | 1846 | 1.3 | ||
| 120 | 1678 | 0.308 | 1644 | 2.0 | ||
| 135 | 1354 | 0.214 | 1286 | −5.0 | ||
| 145 | 1030 | 0.154 | 1004 | −2.5 | ||
| 155 | 672 | 0.095 | 676 | 0.6 | ||
| 160 | 471 | 0.065 | 491 | 4.2 | ||
| 170 | 462 | 0.009 | 74 | −84 | ||
| Phenolic after thermo- relaxation | 25 | 2515 | 1.6 | 1 | - | - |
| 80 | 2375 | 0.729 | 2362 | −0.5 | ||
| 100 | 2250 | 0.641 | 2232 | −1.2 | ||
| 120 | 2130 | 0.557 | 2089 | −1.9 | ||
| 140 | 2085 | 0.478 | 1931 | −7.3 | ||
| 160 | 1900 | 0.402 | 1756 | −7.5 | ||
| 200 | 1486 | 0.261 | 1340 | −9.8 | ||
| 220 | 1200 | 0.210 | 1155 | −3.8 | ||
| 230 | 1045 | 0.178 | 1022 | −2.2 |
| Compound | Temperature, °C | Efact, MPa | S, J/J | kpl | Ecalc, MPa | % Derivation |
|---|---|---|---|---|---|---|
| Epoxy-phenolic before thermo-relaxation | 25 | 3440 | 4.7 | 1 | - | - |
| 85 | 1603 | 0.138 | 1528 | −4.7 | ||
| 90 | 763 | 0.072 | 968 | 26.8 | ||
| 95 | 455 | 0.008 | 139 | −69 | ||
| 100 | 300 | - | - | - | ||
| Epoxy-phenolic after thermo-relaxation | 25 | 3510 | 2.3 | 1 | - | - |
| 85 | 3050 | 0.578 | 3071 | 0.7 | ||
| 90 | 2960 | 0.546 | 3010 | 1.7 | ||
| 100 | 2920 | 0.483 | 2887 | −0.8 | ||
| 110 | 2770 | 0.423 | 2765 | −0.2 | ||
| 120 | 2390 | 0.364 | 2420 | 1.2 | ||
| 130 | 2170 | 0.305 | 2190 | 0.9 | ||
| 140 | 1935 | 0.249 | 1580 | −18.3 | ||
| 160 | 1320 | 0.141 | 1147 | −13.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korolev, A.; Mishnev, M.; Vatin, N.I.; Ignatova, A. Prolonged Thermal Relaxation of the Thermosetting Polymers. Polymers 2021, 13, 4104. https://doi.org/10.3390/polym13234104
Korolev A, Mishnev M, Vatin NI, Ignatova A. Prolonged Thermal Relaxation of the Thermosetting Polymers. Polymers. 2021; 13(23):4104. https://doi.org/10.3390/polym13234104
Chicago/Turabian StyleKorolev, Alexander, Maxim Mishnev, Nikolai Ivanovich Vatin, and Anastasia Ignatova. 2021. "Prolonged Thermal Relaxation of the Thermosetting Polymers" Polymers 13, no. 23: 4104. https://doi.org/10.3390/polym13234104
APA StyleKorolev, A., Mishnev, M., Vatin, N. I., & Ignatova, A. (2021). Prolonged Thermal Relaxation of the Thermosetting Polymers. Polymers, 13(23), 4104. https://doi.org/10.3390/polym13234104