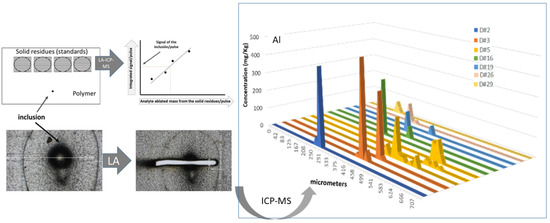
Localized Quantitative Analysis of Polymeric Films through Laser Ablation–Inductively Coupled Plasma Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Laser Ablation System
- -
- The laser cabinet including the active laser medium, all optics apertures, the lighting units, a charge-coupled device camera (CCD) that allowed real-time observation of the ablation process and the ablation cell placed on an adjustable XYZ platform.
- -
- The cooler or power supply that circulated water through the laser head and provided the power necessary for the laser.
- -
- Computer that controlled the sample position, laser firing, camera options, sample cell illumination and gas flow rate by means of the DigilazTM G2 sotfware.
3. Results
3.1. Results for Film # 1
3.2. Results for Film # 2
3.3. Results for Film # 3
4. Discussion
4.1. Development of a Localized Quantitative Analysis Method
4.2. Application of the E-DDCA Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fryer, B.J.; Jackson, S.E.; Longerich, H.P. The design, operation and role of the laser-ablation microprobe coupled with an inductively coupled plasma-mass spectrometer (LAM-ICP-MS) in the earth sciences. Can. Mineral. 1995, 33, 303–312. [Google Scholar]
- Günther, D.; Hattendorf, B. Solid sample analysis using laser ablation inductively coupled plasma mass spectrometry. Trends Anal. Chem. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Gray, A.L. Solid sample introduction by laser ablation for inductively coupled plasma source mass spectrometry. Analyst 1985, 110, 551–556. [Google Scholar] [CrossRef]
- Feldmann, J.; Kindness, A.; Ek, P. Laser ablation of soft tissue using a cryogenically cooled ablation cell. J. Anal. At. Spectrom. 2002, 17, 813–818. [Google Scholar] [CrossRef]
- Becker, J.S.; Zoriy, M.; Matusch, A.; Wu, B.; Salber, D.; Palm, C.; Becker, J.S. Bioimaging of metals by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Mass Spectrom. Rev. 2010, 29, 156–175. [Google Scholar] [CrossRef]
- Becker, J.S.; Matusch, A.; Becker, J.S.; Wu, B.; Palm, C.; Becker, A.J.; Salber, C. Mass spectrometric imaging (MSI) of metals using advanced BrainMet techniques for biomedical research. Int. J. Mass Spectrom. 2011, 307, 3–15. [Google Scholar] [CrossRef]
- Barca, C.; Miriello, D.; Pecci, A.; Barba, L.; Ortiz, A.; Manzanilla, L.R.; Blancas, J.; Crisci, G.M. Provenance of glass shards in archaeological lime plasters by LA-ICP-MS: Implications for the ancient routes from the Gulf of Mexico to Teotihuacan in Central Mexico. J. Arch. Sci. 2013, 40, 3999–4008. [Google Scholar] [CrossRef]
- Rauch, S.; Hemond, H.F. Sediment-based evidence of platinum concentration changes in an urban lake near Boston, Massachusetts. Environ. Sci. Technol. 2003, 37, 3283–3288. [Google Scholar] [CrossRef]
- Garcia, C.C.; Lindner, H.; Niemax, K. Transport efficiency in femtosecond laser ablation inductively coupled plasma mass spectrometry applying ablation cells with short and long washout times. Spectrochim. Acta Part B 2007, 62, 13–19. [Google Scholar] [CrossRef]
- Müller, W.; Shelley, M.; Miller, P. Initial performance metrics of a new custom-designed ArF Excimer LA-ICP-MS system coupled to a two-volume laser-ablation cell. J. Anal. At. Spectrom. 2009, 24, 209–214. [Google Scholar] [CrossRef]
- Hoffmann, E.; Liidke, H.; Scholze, H.; Stephanowitz, H. Analytical investigations of tree rings by laser ablation ICP-MS. Fresenius J. Anal. Chem. 1994, 350, 253–259. [Google Scholar] [CrossRef]
- Bellis, D.J.; Satake, K.; McLeod, C.W. A comparison of lead isotope ratios in the bark pockets and annual rings of two beech trees collected in Derbyshire and South Yorkshire, UK. Sci. Total Environ. 2004, 321, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Siebold, M.; Leidich, P.; Bertini, M.; Deflorio, G.; Feldmann, J.; Krupp, E.M.; Halmschlager, E.; Woodward, S. Application of elemental bioimaging using laser ablation ICPMS in forest pathology: Distribution of elements in the bark of Picea sitchensis following wounding. Anal. Bioanal. Chem. 2012, 402, 3323–3331. [Google Scholar] [CrossRef] [PubMed]
- Bellotto, V.R.; Miekeley, N. Improvements in calibration procedures for the quantitative determination of trace elements in carbonate material (mussel shells) by laser ablation ICP-MS. Fresenius’ J. Anal. Chem. 2000, 367, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zoriy, M.; Chen, Y.; Becker, J.S. Imaging of nutrient elements in the leaves of Elsholtzia splendens by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Talanta 2009, 78, 132–137. [Google Scholar] [CrossRef]
- Meharg, A.A.; Lombi, E.; Williams, P.; Scheckel, K.G.; Feldmann, J.; Raab, A.; Zhu, Y.; Islam, R. Speciation and Localization of Arsenic in White and Brown Rice Grains. Environ. Sci. Technol. 2008, 42, 1051–1057. [Google Scholar] [CrossRef]
- Rauch, S.; Hemond, H.F.; Brabander, D.J. High spatial resolution analysis of lake sediment cores by laser ablationinductively coupled plasma-mass spectrometry (LA-ICP-MS). Limnol. Oceanogr. Methods 2006, 4, 268–274. [Google Scholar] [CrossRef]
- Wang, Y.W.; Specht, A.; Horst, W.J. Stable isotope labelling and zinc distribution in grains studied by laser ablation ICP-MS in an ear culture system reveals zinc transport barriers during grain filling in wheat. New Phytol. 2011, 189, 428–437. [Google Scholar] [CrossRef]
- Resano, M.; Perez-Arantegui, J.; Garcia-Ruiz, E.; Vanhaecke, F. Laser ablation-inductively coupled plasma mass spectrometry for the fast and direct characterization of antique glazed ceramics. J. Anal. At. Spectrom. 2005, 20, 508–514. [Google Scholar] [CrossRef]
- Latkoczy, C.; Muller, Y.; Schmutz, P.; Gunther, D. Quantitative element mapping of Mg alloys by laser ablation ICP-MS and EPMA. Appl. Surf. Sci. 2005, 252, 127–132. [Google Scholar] [CrossRef]
- Villaseñor, A.; Boccongeli, M.; Todolí, J.L. Quantitative elemental analysis of polymers through laser ablation—Inductively coupled plasma by using a dried droplet calibration approach, DDCA. J. Anal. At. Spectrom. 2018, 33, 1173–1183. [Google Scholar] [CrossRef] [Green Version]
- Wolf, R.E.; Thomas, C.; Bohlke, A. Analytical determination of metals in industrial polymers by laser ablation ICP-MS. Appl. Surf. Sci. 1998, 127–129, 299–303. [Google Scholar] [CrossRef]
- Villaseñor, A.; Greatti, C.; Boccongelli, M.; Todolí, J.L. A dried droplet calibration approach for the analysis of solid samples through laser ablation—Inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2017, 32, 587–596. [Google Scholar] [CrossRef] [Green Version]







| Variable | |
|---|---|
| Scan rate (μm/s) | 10 |
| Laser beam diameter (μm) | 50 |
| Spot frequency (Hz) | 20 |
| Laser shot energy (mJ) | 2.7 |
| Laser fluence (J/cm2) | 7.15 |
| Wavelength (nm) | 213 |
| Helium gas flow rates (mL/min) | 500/300 |
| Carrier gas (L/min) Ar added to the aerosol leaving the ablation cell | 0.03 |
| Variable | |
|---|---|
| RF power (KW) | 1.55 |
| Cell Collision (He) (mL/min) | 3.0 |
| Plasma gas flow rate (L/min) | 15.0 |
| Auxiliar gas flow rate (L/min) | 0.9 |
| Integration time/mass s−1 | 0.1 |
| Nuclides measured | 11B, 12C, 13C, 27Al, 24Mg, 28Si, 31P, 40Ar 45Sc 47Ti, 51V, 52Cr, 55Mn, 56Fe, 59Co, 60Ni, 63Cu, 66Zn 75As, 81Br, 88Sr, 90Zr, 95Mo, 111Cd, 107Ag, 121Sb, 136Ba,202Hg, 208Pb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villaseñor, Á.; Sánchez, R.; Boccongelli, M.; Todolí, J.-L. Localized Quantitative Analysis of Polymeric Films through Laser Ablation–Inductively Coupled Plasma Mass Spectrometry. Polymers 2021, 13, 345. https://doi.org/10.3390/polym13030345
Villaseñor Á, Sánchez R, Boccongelli M, Todolí J-L. Localized Quantitative Analysis of Polymeric Films through Laser Ablation–Inductively Coupled Plasma Mass Spectrometry. Polymers. 2021; 13(3):345. https://doi.org/10.3390/polym13030345
Chicago/Turabian StyleVillaseñor, Ángela, Raquel Sánchez, Marina Boccongelli, and José-Luis Todolí. 2021. "Localized Quantitative Analysis of Polymeric Films through Laser Ablation–Inductively Coupled Plasma Mass Spectrometry" Polymers 13, no. 3: 345. https://doi.org/10.3390/polym13030345
APA StyleVillaseñor, Á., Sánchez, R., Boccongelli, M., & Todolí, J. -L. (2021). Localized Quantitative Analysis of Polymeric Films through Laser Ablation–Inductively Coupled Plasma Mass Spectrometry. Polymers, 13(3), 345. https://doi.org/10.3390/polym13030345