Corn Starch/Chitosan Nanoparticles/Thymol Bio-Nanocomposite Films for Potential Food Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cherry Tomato Samples
2.3. Synthesis of Chitosan Nanoparticles (CNP)
2.4. Preparation of the Films
2.5. Characterization on Optical Properties of the Films
2.6. Mechanical Properties
2.7. Water Vapor Permeability
2.8. Demonstration on the Application of the Films as Food Packaging Materials
2.8.1. Firmness
2.8.2. Weight Loss
2.8.3. Shelf-Life Observation
2.9. Statistical Analysis
3. Results and Discussion
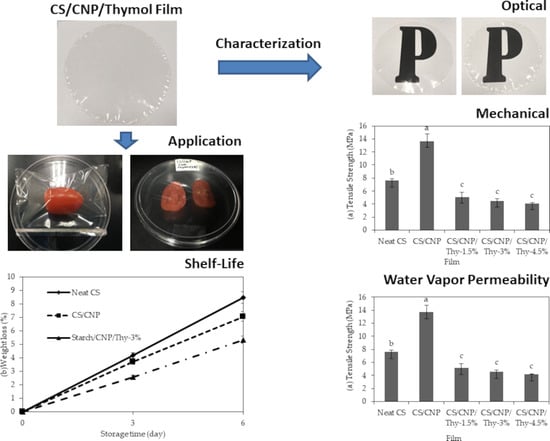
3.1. Optical Properties
3.2. Mechanical Properties
3.3. Water Vapor Permeability
3.4. Demonstration on the Application of CS/CNP/Thy Bio-Nanocomposite Films as Food Packaging
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Othman, S.H.; Kechik, N.R.A.; Shapi’i, R.A.; Talib, R.A.; Tawakkal, I.S.M.A. Water sorption and mechanical properties of starch/chitosan nanoparticle films. J. Nanomater. 2019, 2019, 3843949. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Sharma, V.P. Integrated plastic waste management: Environmental and improved health approaches. Procedia Environ. Sci. 2016, 35, 692–700. [Google Scholar] [CrossRef]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Physical, thermal, morphological, and tensile properties of cornstarch-based films as affected by different plasticizers. Int. J. Food Prop. 2019, 22, 925–941. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Flores, S.; Famá, L.; Rojas, A.M.; Goyanes, S.; Gerschenson, L. Physical properties of tapioca-starch edible films: Influence of film making and potassium sorbate. Food Res. Int. 2007, 40, 257–265. [Google Scholar] [CrossRef]
- Fonseca-García, F.; Jiménez-Regalado, E.J.; Aguirre-Loredo, R.Y. Preparation of a novel biodegradable packaging film based on corn starch-chitosan and poloxamers. Carbohydr. Polym. 2020, 251, 117009. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, H.; Kang, S.; Xia, L.; Jiang, S.; Chen, M.; Jiang, S. An active packaging film based on yam starch with eugenol and its application for pork preservation. Food Hydrocoll. 2019, 96, 546–554. [Google Scholar] [CrossRef]
- Luchese, C.L.; Pavoni, J.M.F.; Santos, N.Z.D.; Quines, L.L.; Pollo, L.D.; Spada, J.C.; Tessaro, I.C. Effect of chitosan addition on the properties of films prepared with corn and cassava starches. J. Food. Sci. Technol. 2018, 55, 2963–2973. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Kulawik, P.; Kopel, P. The effect of nanofillers on the functional properties of biopolymer-based films: A review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef] [Green Version]
- Shapi’i, R.A.; Othman, S.H.; Naim, M.N.; Basha, R.K. Effect of initial concentration of chitosan on the particle size of chitosan nanoparticle. Int. J. Nanotechnol. 2019, 16, 680–691. [Google Scholar] [CrossRef]
- Marchese, A.; Erdogan, I.; Daglia, M.; Barbieri, R.; Lorenzo, A.D.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Mohammad, S. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Liang, Y.; Jiang, S.; Yang, L.; Shi, Y.; Guo, S.; Zhang, C. Physical, antioxidant and antimicrobial properties of modified peanut protein isolate based films incorporating thymol. RSC Adv. 2017, 7, 41610–41618. [Google Scholar] [CrossRef] [Green Version]
- Ghasemlou, M.; Aliheidari, N.; Fahmi, R.; Shojaee-Aliabadi, S.; Keshavarz, B.; Cran, M.J.; Khaksar, R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr. Polym. 2013, 98, 1–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordin, N.; Othman, S.H.; Basha, R.K.; Rashid, S.A. Effects of glycerol and thymol on physical, mechanical, and thermal properties of corn starch films. Food Hydrocoll. 2020, 106, 105884. [Google Scholar] [CrossRef]
- Davoodi, M.; Kavoosi, G.; Shakeri, R. Preparation and characterization of potato starch-thymol dispersion and film as potential antioxidant and antibacterial materials. Int. J. Biol. Macromol. 2017, 104, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.P.; Devchand, K.; Kanny, K. Barrier and biodegradable properties of corn starch-derived biopolymer film filled with nanoclay fillers. J. Plast. Film Sheeting 2017, 33, 309–336. [Google Scholar] [CrossRef]
- Shapi’i, R.A.; Othman, S.H.; Naim, M.N.; Basha, R.K. Mechanical properties of tapioca starch-based film incorporated with bulk chitosan and chitosan nanoparticle: A comparative study. Pertanika J. Sci. Technol. 2019, 27, 95–107. [Google Scholar]
- ASTM D882. Standard Test Method Tensile Properties of Thin Plastic Sheeting; ASTM International: Pennsylvania, PA, USA, 2018; Available online: www.astm.org (accessed on 1 January 2020).
- ASTM E96. Standard Test Method for Water Vapor Transmission of Materials; ASTM International: Pennsylvania, PA, USA, 2016; Available online: www.astm.org (accessed on 1 January 2020).
- Shapi’i, R.A.; Othman, S.H. Effect of concentration of chitosan on the mechanical, morphological and optical properties of tapioca starch film. Int. Food Res. J. 2016, 23, 187–193. Available online: http://www.ifrj.upm.edu.my/23%20(06)%202016%20supplementary/(27)%20IFRJ-16271%20Othman.pdf (accessed on 1 January 2020).
- Mehdizadeh, T.; Tajik, H.; Rohani, S.M.R.; Oromiehie, A.R. Antibacterial, antioxidant and optical properties of edible starch-chitosan composite film containing Thymus kotschyanus essential oil. Vet. Res. Forum 2012, 3, 167–173. Available online: www.ncbi.nlm.nih.gov/pmc/articles/PMC4299978/ (accessed on 1 January 2020).
- Wang, Y.; Zhang, R.; Ahmed, S.; Qin, W.; Liu, Y. Preparation and characterization of corn starch bio-active edible packaging films based on zein incorporated with orange-peel oil. Antioxidants 2019, 8, 391. [Google Scholar] [CrossRef] [Green Version]
- Rosa, C.G.; Sganzerla, W.G.; Maciel, M.V.O.B.; Melo, A.P.Z.; Almeida, A.R.; Nunes, M.R.; Bertoldi, F.C.; Barretoa, P.L.M. Development of poly (ethylene oxide) bioactive nanocomposite films functionalized with zein nanoparticles. Colloids Surf. A 2020, 586, 124268. [Google Scholar] [CrossRef]
- Song, X.; Cheng, L.; Tan, L. Edible iron yam and maize starch convenient food flavoring packaging films with lemon essential oil as plasticization. Food Sci. Technol. 2019, 2061, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Melo-Banda, J.A.; Páramo-García, U.; Paraguay-Delgado, F.; García-Alamilla, R.; Martínez-Hernández, A.L.; Zapién-Castillo, S. Chitosan-starch films with natural extracts: Physical, chemical, morphological and thermal properties. Materials 2018, 11, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorevice, M.V.; Otoni, C.G.; Moura, M.R.; Mattoso, L.H.C. Chitosan nanoparticles on the improvement of thermal, barrier, and mechanical properties of high- and low-methyl pectin films. Food Hydrocoll. 2016, 52, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Chang, P.R.; Yang, J.; Yu, J. Preparation and properties of glycerol plasticized-pea starch/zinc oxide-starch bionanocomposites. Carbohydr. Polym. 2009, 75, 472–478. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Phase transitions in starch based films containing fatty acids. Effect on water sorption and mechanical behavior. Food Hydrocoll. 2013, 30, 408–418. [Google Scholar] [CrossRef]
- Tawakkal, I.S.M.A.; Cran, M.J.; Bigger, S.W. The influence of chemically treated natural fibers in poly(lactic acid) composites containing thymol. Polym. Compos. 2018, 39, 1261–1272. [Google Scholar] [CrossRef] [Green Version]
- Moura, M.R.; Lorevice, M.V.; Mattoso, L.H.C.; Zucolotto, V. Highly stable, edible cellulose films incorporating chitosan nanoparticles. J. Food Sci. 2011, 76, 25–29. [Google Scholar] [CrossRef]
- Rodrigues, C.; Mello, J.M.M.; Dalcanton, F.; Lusitâneo, D.; Macuvele, D.L.P.; Padoin, N.; Fiori, M.A.; Soares, C.; Riella, H.G. Mechanical, thermal and antimicrobial properties of chitosan-based-nanocomposite with potential applications for food packaging. J. Polym. Environ. 2020, 28, 1216–1236. [Google Scholar] [CrossRef]
- Robledo, N.; Vera, P.; López, L.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Thymol nanoemulsions incorporated in quinoa protein/chitosan edible films; antifungal effect in cherry tomatoes. Food Chem. 2018, 246, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J. Mater. Sci. Mater. Med. 2010, 21, 2227–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelissari, F.M.; Grossmann, M.V.E.; Yamashita, F.; Pineda, E.A.G. Antimicrobial, mechanical, and barrier properties of cassava starch−chitosan films incorporated with oregano essential oil. J. Agr. Food Chem. 2009, 57, 7499–7504. [Google Scholar] [CrossRef] [PubMed]
- Akter, N.; Tuhin, M.; Fahmida, P. Thermomechanical, barrier, and morphological properties of chitosan-reinforced starch-based biodegradable composite films. J. Thermoplast. Compos. Mater. 2012, 1–16. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Pertile, G.; Pieczywek, P.M.; Cieślak, K.J.; Zdunek, A.; Frąc, M. Influence of chitosan addition on the mechanical and antibacterial properties of carrot cellulose nanofibre film. Cellulose 2019, 26, 9613–9629. [Google Scholar] [CrossRef] [Green Version]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/organoclay bionanocomposites impregnated with thymol and cinnamaldehyde by supercritical impregnation for active and sustainable food packaging. Compos. B Eng. 2019, 176, 107336. [Google Scholar] [CrossRef]
- Kavoosi, G.; Dadfar, S.M.M.; Purfard, A.M. Mechanical, physical, antioxidant, and antimicrobial properties of gelatin films incorporated with thymol for potential use as nano wound dressing. J. Food Sci. 2013, 78, 244–250. [Google Scholar] [CrossRef]
- Tavakoli, S.; Yassa, N.; Delnavazi, M.R.; Akhbari, M.; Hadjiakhoondi, A.; Hajimehdipoor, H.; Khalighi-Sigaroodi, F.; Hajiaghaee, R. Chemical composition and biological activities of the essential oils from different parts of Ferulago trifida Boiss. J. Essent. Oil Res. 2017, 29, 407–419. [Google Scholar] [CrossRef]
- Khairuddin, N.; Muhamad, I.I.; Abd Rahman, W.A.W.; Siddique, B.M. Physicochemical and thermal characterization of hydroxyethyl cellulose—Wheat starch based films incorporated thymol intended for active packaging. Sains Malays. 2020, 49, 323–333. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, Y.; Yuan, M.; Li, L.; Chen, H.; Cao, J.; Yang, J. Characterization of an antimicrobial poly(lactic acid) film prepared with poly(ε-caprolactone) and thymol for active packaging. Polym. Adv. Technol. 2014, 25, 948–954. [Google Scholar] [CrossRef]
- Shen, X.L.; Wu, J.M.; Chen, Y.; Zhao, G. Antimicrobial and physical properties of sweet potato starch films incorporated with potassium sorbate or chitosan. Food Hydrocoll. 2010, 24, 285–290. [Google Scholar] [CrossRef]
- Moey, S.W.; Abdullah, A.; Ahmad, I. Effect of Cinnamomum zeylanicum essential oil on the physical and mechanical properties of edible films from Kappaphycus alvarezii. Malays. Appl. Biol. 2018, 47, 197–203. [Google Scholar]
- Abiso, E.; Satheesh, N.; Hailu, A. Effect of storage methods and ripening stages on postharvest quality of tomato (Lycopersicom esculentum Mill) cv. Chali. Annals. Food Sci. Technol. 2015, 16, 127–137. [Google Scholar]
- Amal, S.H.A.; El-Mogy, M.M.; Aboul-Anean, H.E.; Alsanius, B.W. Improving strawberry fruit storability by edible coating as a carrier of thymol or calcium chloride. J. Hort. Sci. Ornam. Plants 2010, 2, 88–97. [Google Scholar]
- Correa-Pacheco, Z.N.; Angel, M.; Bautista-Ba, S. The effect of nanostructured chitosan and chitosan-thyme essential oil coatings on colletotrichum gloeosporioides growth in vitro and on cv hass avocado and fruit quality. J. Phytopathol. 2017, 165, 297–305. [Google Scholar] [CrossRef]
- Rahimi, R.; Valizadehkaji, B.; Khadivi, A.; Shahrjerdi, I. Effect of chitosan and thymol essential oil on quality maintenance and shelf life extension of peach fruits cv. ‘Zaferani’. J. Hortic. Postharvest Res. 2019, 2, 143–156. [Google Scholar] [CrossRef]
- Qin, Y.; Zhuang, Y.; Wu, Y.; Li, L. Quality evaluation of hot peppers stored in biodegradable poly(lactic acid)-based active packaging. Sci. Hortic. 2016, 202, 1–8. [Google Scholar] [CrossRef]
- Ramos, M.; Beltran, A.; Valdes, A.; Peltzer, M.A.; Jimenez, A.; Garrigos, M.C.; Zaikov, G.E. Carvacrol and thymol for fresh food packaging. J. Bioequival. Bioavailab. 2013, 5, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Medina, E.; Caro, N.; Abugoch, L.; Gamboa, A.; Díaz-Dosque, M.; Tapia, C. Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J. Food Eng. 2019, 240, 191–198. [Google Scholar] [CrossRef]
- Antoniou, J.; Liu, F.; Majeed, H.; Zhong, F. Characterization of tara gum edible films incorporated with bulk chitosan and chitosan nanoparticles: A comparative study. Food Hydrocoll. 2015, 44, 309–319. [Google Scholar] [CrossRef]
- Sun, Q.; Xi, T.; Li, Y.; Xiong, L. Characterization of corn starch films reinforced with CaCO3 nanoparticles. PLoS ONE 2014, 9, e106727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.S.; Singh, S.; Lee, Y.S. Characterization of edible film containing essential oils in hydroxypropyl methylcellulose and its effect on quality attributes of ‘Formosa’ plum (Prunus salicina L.). LWT Food Sci. Technol. 2016, 70, 213–222. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I.; El-Nouby, M.A.M.; Ismail, R.I.A.; Taktak, N.E.M. Strawberry shelf life, composition, and enzymes activity in response to edible chitosan coatings strawberry shelf life, composition, and enzymes activity in response to edible chitosan coatings. Int. J. Fruit Sci. 2017, 17, 117–136. [Google Scholar] [CrossRef]
- Lustriane, C.; Dwivany, F.M.; Suendo, V.; Reza, M. Effect of chitosan and chitosan-nanoparticles on post harvest quality of banana fruits. J. Plant Biotechnol. 2018, 45, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Xu, Q.; Li, X.; Chen, C.; Ma, L.; Li, S.; Che, Z.; Lin, H. Chitosan-based coating with antimicrobial agents: Preparation, property, mechanism, and application effectiveness on fruits and vegetables. Int. J. Polym. Sci. 2016, 2016, 4851730. [Google Scholar] [CrossRef] [Green Version]
- Shapi’i, R.A.; Othman, S.H.; Nordin, N.; Basha, R.K.; Naim, M.N. Antimicrobial properties of starch films incorporated with chitosan nanoparticles: In vitro and in vivo evaluation. Carbohydr. Polym. 2020, 230, 115602. [Google Scholar] [CrossRef]
- Marino, M.; Bersani, C.; Comi, G. Antimicrobial activity of the essential oils of Thymus vulgaris L. measured using a bioimpedimetric method. Int. J. Food Microbiol. 2001, 67, 187–195. [Google Scholar] [CrossRef]
- Shah, B.; Davidson, P.M.; Zhong, Q. Nanocapsular dispersion of thymol for enhanced dispersibility and increased antimicrobial effectiveness against Escherichia coli O157:H7 and Listeria monocytogenes in model food systems. Appl. Environ. Microbiol. 2012, 78, 8448–8453. [Google Scholar] [CrossRef] [Green Version]







| Percentage Weight of Thymol/Weight of Starch (w/w%) | Weight of Chitosan (g) | Weight of Thymol (g) | Weight of Tween 80 (µL) | Weight of TPP (g) |
|---|---|---|---|---|
| 0 | 0.45 | 0 | 0 | 0.09 |
| 1.5 | 0.45 | 0.045 | 275 | 0.09 |
| 3.0 | 0.45 | 0.09 | 275 | 0.09 |
| 4.5 | 0.45 | 0.135 | 275 | 0.09 |
| Concentration of Thymol (% w/w) | L* | a* | b* | Total Color Difference (ΔE) | Opacity (A600/mm) |
|---|---|---|---|---|---|
| 0% | 93.77 ± 0.14 a | −1.10 ± 0.02 ab | 3.74 ± 0.19 b | 0 | 0.80 ± 0.04 b |
| 1.5% | 92.99 ± 0.03 b | −1.04 ± 0.05 a | 3.60 ± 0.21 b | 0.81 ± 0.03 b | 0.81 ± 0.02 b |
| 3.0% | 92.95 ± 0.10 b | −1.12 ± 0.07 ab | 3.62 ± 0.13 b | 0.84 ± 0.10 b | 0.87 ± 0.01 b |
| 4.5% | 92.54 ± 0.05 c | −1.19 ± 0.03 b | 4.24 ± 0.06 a | 1.33 ± 0.03 a | 1.06 ± 0.07 a |
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|---|---|---|---|
| (a) |  |  |  |  |  |  |  |  |
| (b) |  |  |  |  |  |  |  |  |
| (c) |  |  |  |  |  |  |  |  |
| (d) |  |  |  |  |  |  |  |  |
| (e) |  |  |  |  |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, S.H.; Othman, N.F.L.; Shapi’i, R.A.; Ariffin, S.H.; Yunos, K.F.M. Corn Starch/Chitosan Nanoparticles/Thymol Bio-Nanocomposite Films for Potential Food Packaging Applications. Polymers 2021, 13, 390. https://doi.org/10.3390/polym13030390
Othman SH, Othman NFL, Shapi’i RA, Ariffin SH, Yunos KFM. Corn Starch/Chitosan Nanoparticles/Thymol Bio-Nanocomposite Films for Potential Food Packaging Applications. Polymers. 2021; 13(3):390. https://doi.org/10.3390/polym13030390
Chicago/Turabian StyleOthman, Siti Hajar, Nur Fitrah Liyana Othman, Ruzanna Ahmad Shapi’i, Siti Hajar Ariffin, and Khairul Faezah Md. Yunos. 2021. "Corn Starch/Chitosan Nanoparticles/Thymol Bio-Nanocomposite Films for Potential Food Packaging Applications" Polymers 13, no. 3: 390. https://doi.org/10.3390/polym13030390
APA StyleOthman, S. H., Othman, N. F. L., Shapi’i, R. A., Ariffin, S. H., & Yunos, K. F. M. (2021). Corn Starch/Chitosan Nanoparticles/Thymol Bio-Nanocomposite Films for Potential Food Packaging Applications. Polymers, 13(3), 390. https://doi.org/10.3390/polym13030390