Evaluation of Novel Ornamental Cladding Resistance, Comprised of GFRP Waste and Polyester Binder, within an Acid Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Manufacturing Methodology of New Composite
2.3. Compressive Mechanical Tests
2.4. Chemical Substances Influence Evaluation of the Composite Plate
- Case 1: Rainwater, pH = 6 (RW);
- Case 2: Solution of 1:1: 5 mL acetic acid + 5 mL distilled water and ammonia (18 drops) for adjustment, pH = 3.5 (AA);
- Case 3: Solution of 18 mL distilled water + 3 mL concentrated sulfuric acid + 2 mL concentrated nitric acid + ammonia, until pH = 2 (SNA).
2.5. Morphological Analyses
2.6. X-ray Diffraction Analysis (XRD)
2.7. Mineralogical Optical Microscopy in Polarized Light with Crossed Nicols (CPOM)
3. Results and Discussion
- -
- Rainwater (M = 128.260, SD = 66.643); t (4) = 0.172, p = 0.871;
- -
- Acetic acid solution (M = 188.200, SD =119.144); t (4) = 1.186, p = 0.301;
- -
- Nitric acid solution (M = 247.200, SD = 152.165); t (4) = 1.820, p = 0.142.
- -
- Rainwater (M = 164.40, SD = 82.28); t (4) = 0.236, p = 0.8249;
- -
- Acetic acid solution (M = 237.20, SD = 142.59); t (4) = 1.252, p = 0.279;
- -
- Nitric acid solution (M = 307.00, SD = 168.64); t (4) = 2.015, p = 0.114.
- -
- Rainwater (M = 250.180, SD = 143.445); t (4) = 1.183, p = 0.912;
- -
- Acetic acid solution (M = 315.600, SD = 96.984); t (4) = 0.955, p = 0.393;
- -
- Sulfuric + nitric acid solution (M = 333.000, SD = 117.792); t (4) = 1.138, p = 0.319.
- -
- Rainwater (M = 302.660, SD = 163.651); t (4) = 0.125, p = 0.906;
- -
- Acetic acid solution (M = 410.800, SD = 136.844); t (4) = 1.304, p = 0.262;
- -
- Sulfuric + nitric acid solution (M = 409.000, SD = 140.260); t (4) = 1.257, p = 0.277.
4. Conclusions
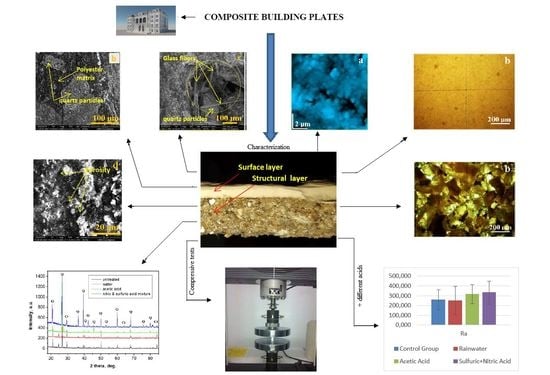
- This paper presents a study regarding the evaluation of novel ornamental cladding resistance, comprised of GFRP waste and a polyester matrix. The wet material is pressed into a mold, and in the end, an ornamental plate that copies natural stone was obtained.
- The results of mechanical compression tests of the proposed material indicate 78 MPa. This value is comparable to C70/85 concrete. The proposed material solves a very important problem for composite factories. They accumulate large amounts of waste that are difficult to manage. Integrating GFRP waste and obtaining new materials can be a solution. The application of new materials also solves an important environmental problem. This may eliminate the environmental impact of stone quarries.The results of the performed analyses on the material regarding behavior upon possible chemical attacks when exposed to the environment show a very good behavior.The initial structures of both the composite plate and the ones that were acid attacked (acetic and nitric acids, which can be found in the mixing of rainwater with environmental pollution) were analyzed.
- In order to check the surface modifications, SEM and AFM image analyses were conducted for each area of the prepared samples and for the interface between both components.
- Using software, the surface roughness values Ra and Rq were determined for each image. An independent samples t-test was conducted to compare surface roughness in untreated and treated surfaces for both situations.Following the statistical analysis of the roughness for both Ra and Rq cases, the obtained results clearly showed that there is no significant difference between the roughness of the control group surface (untreated) and the roughness of each sample treated with different substances for the exterior surface for the level α = 0.05 (p-value > 0.05 was obtained for all tests). This was also found in the case of the interior surface, meaning that rainwater does not affect the structure of the plate even if it is acid rain, or the plates are used in high-pollution environments. Components such as glass, polymer, and quartz particles are neutral from a chemical point of view.
- CPOM microscopy showed that even if calcite at micro- and nano-structural levels is soluble in acid solutions (which can appear due to rain), the presence of the polymer within the building plates reduces the erosion risk.SEM images also demonstrated that in the created porosity with different acid solutions, there was no capillarity to permit water infiltration, only some individual surface pores.
- This paper highlights the resistance properties of these composite plates in exterior environmental conditions. Taking into consideration that the exterior and interior surfaces are not significantly affected by these acid attacks, it can be concluded that the presented building plates are suitable for exterior building walls from a chemical attack point of view and provide resistance against compressive stresses. Therefore, these plates can be manufactured and used for cladding buildings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Striani, R.; Corcione Esposito, C.; Dell’Anna Muia, G.; Frigione, M. Durability of a sunlight-curable organic–inorganic hybrid protective coating for porous stones in natural and artificial weathering conditions. Prog. Org. Coat. 2016, 101, 1–14. [Google Scholar] [CrossRef]
- Eyssautier-Chuinea, S.; Calandra, I.; Vaillant-Gaveau, N.; Fronteau, G.; Thomachot-Schneider, C.; Hubert, J.; Pleck, J.; Gommeaux, M. A new preventive coating for building stones mixing a water repellent and an eco-friendly biocide. Prog. Org. Coat. 2018, 120, 132–142. [Google Scholar] [CrossRef]
- Pascal, R. La Sculpture—Méthod eset Matériaux Nouveaux; Dessain et Tolra: Paris, France, 2001; p. 64. [Google Scholar]
- Pascal, R. Le Moulage; Dessain et Tolra: Paris, France, 2001; p. 158. [Google Scholar]
- Hoang, Q.V.; Thuy Ninh, N.; Nguyen Vo Hoang, G. The properties of fiber reinforced mortar applied in decorative products. J. Civ. Eng. Res. 2018, 8, 1–8. [Google Scholar]
- Henriques, F.M.A.; Rato, V.M.; Charola, A.E. The influence of grain size distribution on the performance of mortars. In Proceedings of the 10th International Congress on Deterioration and Conservation of Stone, Stockholm, Sweeden, 27 June–2 July 2004; Kwiatkowski, D., Loöfvendahl, R., Eds.; ICOMOS: Stockholm, Sweden, 2004; pp. 1001–1008. [Google Scholar]
- Sebaibi, N.; Benzerzour, M.; Abriak, N.E.; Binetruy, C. Mechanical properties of concrete-reinforced fibre sand powders with crushed thermoset composites: The influence of fibre/matrix interaction. Constr. Build. Mater. 2012, 29, 332–338. [Google Scholar] [CrossRef]
- Park, S.B.; Lee, B.C.; Kim, J.H. Studies on mechanical properties of concrete containing waste glass aggregate. Cem. Concr. Res. 2004, 34, 2181–2189. [Google Scholar] [CrossRef]
- Mohanu, I.; Georgescu, M.; Mohanu, D.; Manolache, L.; Draganoaia, C. Physical-mechanical and aesthetic characteristics of some mortars based on lime-limestone-volcanic tuff. Rev. Rom. Mater. 2011, 41, 332–345. [Google Scholar]
- Papayianni, I.; Stefanidou, M. The role of fines in lime-based mortar’s technology. Rev. Rom. Mater. 2011, 41, 26–29. [Google Scholar]
- Sathishkumar, T.P.; Satheeshkumar, S.; Naveen, J. Glass fiber-reinforced polymer composites—A review. J. Reinf. Plast. Comp. 2014, 33, 1258–1275. [Google Scholar] [CrossRef]
- Aramide, F.O.; Atanda, P.O.; Olorunniwo, O.O. Mechanical properties of a polyester fiber glass composite. Int. J. Compos. Mater. 2012, 2, 147–151. [Google Scholar]
- Alam, S.; Habib, F.; Irfan, M.; Iqbal, W.; Khalid, K. Effect of orientation of glass fiber on mechanical properties of GRP composites. J. Chem.Soc. Pak. 2010, 32, 265–269. [Google Scholar]
- Gan, X.Y. Effect of interface structure on mechanical properties of advanced composite materials. Int. J. Mol.Sci. 2009, 10, 5115–5134. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Akter, R.; Alam, M.Z.; Qadir, M.R.; Begum, M.H.A.; Gafur, M.A. Preparation and characterization of sand reinforced polyester composites. Int. J. Eng. Technol. 2013, 13, 111–118. [Google Scholar]
- Davies, P. Environmental degradation of composites for marine structures: New materials and new applications. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150272. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.C.S.; Ferreira, A.J.M.; Marques, A.T. Effect of natural and artificial weathering on the long-term flexural performance of polymer mortars. Mech. Compos. Mater. 2009, 45, 515–526. [Google Scholar] [CrossRef]
- Araujo, M.E.; Araujo, D.K.; Pereira, D.O.; Ribeiro, C.P.; Melo, J.A.T. Fiber glass wastes/polyester resin composites mechanical properties and water sorption. Polim.Ciencia e Tecno. 2006, 16, 332–335. [Google Scholar] [CrossRef]
- Renaud, M.C.; Greenwood, E.M. Effect of Glass Fibres and Environments on Long-Term Durability of GFRP Composites; Owens Corning Composites Publications: Battice, Belgium, 2009. [Google Scholar]
- Chhibber, R.; Sharma, A.; Mukherjee, A.; Mishnaevsky, L., Jr. Environmental degradation of glass fibre reinforced polymer composite. UpWind 2006, 6, 1–48. [Google Scholar]
- Neale, K.W. FRPs for structural rehabilitation: A survey of recent progress. Progr. Struc. Eng. Mater. 2000, 2, 133–138. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Manalo, A.C.; Ferdous, W.; Zhuge, Y.; Vijay, P.V.; Pettigrew, J. Experimental and numerical evaluations on the behaviour of structures repaired using prefabricated FRP composites jacket. Eng. Struct. 2020, 210, 110358. [Google Scholar] [CrossRef]
- Di Tommaso, A.; Gentilini, C.; Castellazzi, G. Durability analysis for FRP and SRG composites in Civil Applications. Key Eng. Mater. 2014, 624, 421–428. [Google Scholar]
- Polini, W.; Sorrentino, L.; Turchetta, S.; Fiorini, M. Polymeric composite laminate to increase the performance of natural stones. Int. J. Eng. Technol. 2015, 7, 453–460. [Google Scholar]
- Bellini, C.; Polini, W.; Sorrentino, L.; Turchetta, S. Mechanical performances increasing of natural stones by GFRP sandwich structures. Proc. Struct. Integr. 2018, 9, 179–185. [Google Scholar]
- Sorrentino, L.; Bellini, C.; Polini, W.; Turchetta, S. Performance index of natural stones-GFRP hybrid structures. Frat. Integrità Strutt. 2018, 12, 285–294. [Google Scholar] [CrossRef]
- Al-Rubaye, M.; Manalo, A.; Alajarmeh, O.; Ferdous, W.; Lokuge, W.; Benmokrane, B.; Edoo, A. Flexural behaviour of concrete slabs reinforced with GFRP bars and hollow composite reinforcing systems. Compos. Struct. 2020, 236, 111836. [Google Scholar] [CrossRef] [Green Version]
- Pickering, S.J. Recycling technologies for thermoset composite materials—Current status. Compos Part A Appl. S 2006, 37, 1206–1215. [Google Scholar] [CrossRef]
- Conroy, A.; Halliwell, S.; Reynolds, T. Composites recycling in the construction industry. Compos Part A Appl. S 2006, 37, 1216–1222. [Google Scholar] [CrossRef]
- Meira Castro, A.C.; Ribeiro, M.C.S.; Santos, J.; Meixedo, J.P.; Silva, F.J.G.; Fiúza, A.; Dinis, M.L.; Alvim, M.R. Sustainable waste recycling solution for the glass fibre reinforced polymer composite materials industry. Constr. Build. Mater. 2013, 45, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Oliveux, G.; Dandy, O.L.; Leeke, A.G. Current status of recycling of fibre reinforced polymers: Review of technologies, reuse and resulting properties. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, S.; Pinho, S.T. Recycling carbon fibre reinforced polymers for structural applications: Technology review and market outlook. Waste Manag. 2011, 31, 378–392. [Google Scholar] [CrossRef] [Green Version]
- Ceclan, V.; Bere, P.; Borzan, M.; Grozav, S.; Borzan, C. Development of environmental technology for carbon fibre reinforced materials recycling. Mater. Plast. 2013, 50, 79–83. [Google Scholar]
- Thomas, C.; Lombillo, I.; Polanco, J.A.; Villegas, L.; Setien, J.; Biezma, M.V. Polymeric and cementitious mortars for the reconstruction of natural stone structures exposed to marine environments. Compos. Part B 2010, 41, 663–672. [Google Scholar] [CrossRef]
- Nobili, A. Durability assessment of impregnated Glass Fabric Reinforced Cementitious Matrix (GFRCM) composites in the alkaline and saline environments. Constr. Build. Mater. 2016, 105, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Amaro, A.M.; Reis, P.N.B.; Neto, M.A.; Louro, C. Effects of alkaline and acid solutions on Glass/Epoxy Composites. Polym. Degrad. Stabil. 2013, 98, 853–862. [Google Scholar] [CrossRef]
- Reis, J.M.L. Mechanical characterization of polymer mortars exposed to degradation solutions. Constr. Build. Mater. 2009, 23, 3328–3331. [Google Scholar] [CrossRef]
- Gorninski, J.P.; Dal Molin, D.C.; Kazmierczak, C.S. Strength degradation of polymer concrete in acidic environments. Cem. Concr. Compos. 2007, 29, 637–645. [Google Scholar] [CrossRef]
- Gutberlet, T.; Hilbig, H.; Beddoe, R.E. Acid attack on hydrated cement—Effect of mineral acids on the degradation process. Cem. Concr. Res. 2015, 74, 35–43. [Google Scholar] [CrossRef]
- Dang, W.; Kubouchi, M.; Maruyama, T.; Sembokuya, H.; Tsudo, K. Decomposition of GFRP in nitric acid and hydrogen peroxide solution for chemical recycling. Prog. Rubber Plast. Re. 2002, 18, 49–67. [Google Scholar] [CrossRef]
- Dang, W.; Kubouchi, M.; Sembokuya, H.; Tsuda, K. Chemical recycling of glass fiber reinforced epoxy resin cured with amine using nitric acid. Polymer 2005, 46, 1905–1912. [Google Scholar] [CrossRef]
- Pavlik, V. Effect of carbonates on the corrosion rate of cement mortars in nitric acid. Cem. Concr. Res 2000, 30, 481–489. [Google Scholar] [CrossRef]
- Pavlík, V. Corrosion of hardened cement paste by acetic and nitric acids part I: Calculation of corrosion depth. Cem. Concr. Res 1994, 24, 551–562. [Google Scholar] [CrossRef]
- Sabău, E.; Bâlc, N.; Bere, P. Method and Composite Material for Ornamental Synthetic Plates. Manufacturing. Patent No. RO 130062, international C04B 14/36 (2006.01), 28 February 2017. [Google Scholar]
- Sabău, E. Recycling of polymeric composite materials. In Product Lifecycle Management—Terminology and Applications; Udroiu, R., Bere, P., Eds.; IntechOpen: London, UK, 2018; pp. 103–121. [Google Scholar]
- Sabău, E.; Udroiu, R.; Bere, P.; Buranský, I.; Miron-Borzan, C.S. A novel polymer concrete composite with GFRP waste: Applications, morphology, and porosity characterization. Appl. Sci. Basel 2020, 10, 2060. [Google Scholar] [CrossRef] [Green Version]
- Mendivil-Escalante, J.M.; Gómez-Soberón, J.M.; Almaral-Sánchez, J.L.; Cabrera-Covarrubias, F.G. Metamorphosis in the porosity of recycled concretes through the use of a recycled polyethylene terephthalate (PET) additive. Correlations between the porous network and concrete properties. Materials (Basel) 2017, 10, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, N.; Abro, A.M.; Purdue, J.D.; Vaidya, U. 13—Thermoplastic composites for bridge structures. In Woodhead Publishing Series in Civil and Structural Engineering, Developments in Fiber-Reinforced Polymer (FRP) Composites for Civil Engineering; Woodhead Publishing: Birmingham, AL, USA; pp. 317–350e.
- Hosu-Prack, A.G.; Petean, I.; Arghir, G.; Bobos, L.D.; Iurcut, I.; Tomoaia-Cotisel, M. Marble nano erosion under acid rain evidenced by atomic force microscopy. Carpath. J. Earth. Env. 2013, 8, 75–82. [Google Scholar]
- Hosu-Prack, A.G.; Petean, I.; Arghir, G.; Muntean, D.F.; Tomoaia-Cotisel, M. Silicates fragmentation a source of atmosphere dispersed nano—particulate matter. Rev. Chim. 2016, 67, 1118–1123. [Google Scholar]













| Sample Notation | Maximum Compressive Load (KN) | Average Compressive Load (KN) | Standard Deviation | Average Compressive Stress (MPa) |
|---|---|---|---|---|
| S1 | 192.6 | |||
| S2 | 188.8 | |||
| S3 | 192.4 | 190.76 | 1.76 | 78.89 |
| S4 | 189.2 | |||
| S5 | 190.8 |
| Measurement Points | Surface Layer | |||
|---|---|---|---|---|
| Roughness in 5 Different Points, Ra (μm) | ||||
| Control Group | Group Test | |||
| Rainwater | Acetic Acid Solution | Sulfuric + Nitric Acid Solution | ||
| 1 | 120.0 | 127.0 | 396.0 | 136.0 |
| 2 | 76.6 | 243.0 | 178.0 | 399.0 |
| 3 | 88.2 | 87.5 | 111.0 | 423.0 |
| 4 | 118.0 | 79.8 | 116.0 | 101.0 |
| 5 | 207.0 | 104.0 | 140.0 | 177.0 |
| Mean | 121.960 | 128.260 | 188.200 | 247.200 |
| Std. Dev. | 51.109 | 66.643 | 119.144 | 152.165 |
| t-value | 0.172762 | 1.186278093 | 1.82045214 | |
| p-value | 0.871228 | 0.301158824 | 0.14280207 | |
| Roughness in 5 Different Points, Rq (μm) | ||||
| 1 | 164.0 | 166.0 | 484.0 | 191.0 |
| 2 | 95.2 | 304.0 | 233.0 | 470.0 |
| 3 | 113.0 | 109.0 | 143.0 | 505.0 |
| 4 | 152.0 | 101.0 | 147.0 | 137.0 |
| 5 | 246.0 | 142.0 | 179.0 | 232.0 |
| Mean | 154.04 | 164.40 | 237.20 | 307.00 |
| Std. Dev. | 58.54 | 82.28 | 142.59 | 168.64 |
| t-value | 0.236 | 1.252 | 2.015 | |
| p-value | 0.8249 | 0.2786 | 0.1141 | |
| Measurement Points | Structural Layer | |||
|---|---|---|---|---|
| Roughness in 5 Different Points, Ra (μm) | ||||
| Control Group | Group Test | |||
| Rainwater | Acetic Acid Solution | Sulfuric + Nitric Acid Solution | ||
| 1 | 309.0 | 264.0 | 463.0 | 298.0 |
| 2 | 193.0 | 455.0 | 363.0 | 297.0 |
| 3 | 164.0 | 165.0 | 275.0 | 244.0 |
| 4 | 219.0 | 293.0 | 246.0 | 286.0 |
| 5 | 411.0 | 73.9 | 231.0 | 540.0 |
| Mean | 259.200 | 250.180 | 315.600 | 333.000 |
| Std. Dev. | 100.728 | 143.445 | 96.984 | 117.792 |
| t-value | 0.118284 | 0.955398328 | 1.137632686 | |
| p-value | 0.911544779 | 0.393479029 | 0.318793636 | |
| Roughness in 5 Different Points, Rq (μm) | ||||
| 1 | 367.0 | 318.0 | 625.0 | 370.0 |
| 2 | 244.0 | 525.0 | 466.0 | 369.0 |
| 3 | 203.0 | 199.0 | 355.0 | 310.0 |
| 4 | 262.0 | 373.0 | 299.0 | 340.0 |
| 5 | 492.0 | 98.3 | 309.0 | 656.0 |
| Mean | 313.600 | 302.660 | 410.800 | 409.000 |
| Std. Dev. | 116.637 | 163.651 | 136.844 | 140.260 |
| t-value | 0.125 | 1.304 | 1.257 | |
| p-value | 0.90643 | 0.262095888 | 0.276984367 | |
| Mineral | Calcite | Quartz |
|---|---|---|
| Formula | CaCO3 | SiO2 |
| Cluster size, µm | 1–5 | 10–400 |
| Color | Yellow | Green-gray |
| Shape | Round, clusters | Round, elongated with sharp edges |
| Crystal Grain Size in the Exterior Area, nm | Crystal Grain Size in the Interior—Porous Area, µm | |||
|---|---|---|---|---|
| XRD | AFM | XRD | AFM | |
| Control group | 68.44 | 73 | 110.68 | 120 |
| Rainwater | 69.84 | 75 | 110.49 | 120 |
| Acetic acid | 71.36 | 80 | 108.78 | 110 |
| Azotic acid | - | - | 109.46 | 115 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabău, E.; Bere, P.; Moldovan, M.; Petean, I.; Miron-Borzan, C. Evaluation of Novel Ornamental Cladding Resistance, Comprised of GFRP Waste and Polyester Binder, within an Acid Environment. Polymers 2021, 13, 448. https://doi.org/10.3390/polym13030448
Sabău E, Bere P, Moldovan M, Petean I, Miron-Borzan C. Evaluation of Novel Ornamental Cladding Resistance, Comprised of GFRP Waste and Polyester Binder, within an Acid Environment. Polymers. 2021; 13(3):448. https://doi.org/10.3390/polym13030448
Chicago/Turabian StyleSabău, Emilia, Paul Bere, Mărioara Moldovan, Ioan Petean, and Cristina Miron-Borzan. 2021. "Evaluation of Novel Ornamental Cladding Resistance, Comprised of GFRP Waste and Polyester Binder, within an Acid Environment" Polymers 13, no. 3: 448. https://doi.org/10.3390/polym13030448
APA StyleSabău, E., Bere, P., Moldovan, M., Petean, I., & Miron-Borzan, C. (2021). Evaluation of Novel Ornamental Cladding Resistance, Comprised of GFRP Waste and Polyester Binder, within an Acid Environment. Polymers, 13(3), 448. https://doi.org/10.3390/polym13030448