Effect of Monochloroacetic Acid on Properties of Carboxymethyl Bacterial Cellulose Powder and Film from Nata de Coco
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Bacterial Cellulose
2.3. Synthesis of Carboxymethyl Cellulose from Bacterial Cellulose (CMCn)
2.4. Color Characteristics
2.5. CMCn Film Preparation
2.6. The Degree of Substitution (DS) of CMCn
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Viscosity
2.9. Scanning Electron Microscopy (SEM)
2.10. Solubility
2.11. Mechanical Properties
2.12. Water Vapor Transmission Rate (WVTR)
2.13. Statistical Analysis
3. Results and Discussion
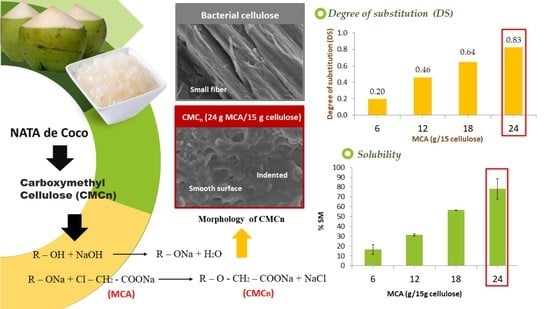
3.1. Degree of Substitution (DS) and Percent Yield of CMCn
3.2. Solubility
3.3. Color
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.5. Effect of Various MCA Levels on Viscosity of CMCn
3.6. Scanning Electron Microscopy of CMCn Powder
3.7. Water Vapor Permeability (WVP)
3.8. Tensile Strength (TS) and Elongation at Break (%EB)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adejoye, O.D.; Adebayo-Toyo, B.C.; Ogunjobi, A.A.; Olaoye, O.A.; Fadahunsi, F.I. Effect of carbon, nitrogen and mineral sources on growth of Pleurotus florida, a Nigeria edible mushroom. Afr. J. Biotechnol. 2006, 5, 1355–1359. [Google Scholar]
- Kurosumi, A.; Sasaki, C.; Yamashita, Y.; Nakamura, Y. Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr. Polym. 2009, 76, 333–335. [Google Scholar] [CrossRef]
- Cannon, R.E.; Anderson, S.M. Biogenesis of bacterial Cellulose. Crit. Rev. Microbiol. 1991, 17, 435–447. [Google Scholar] [CrossRef]
- Chen, S.; Zou, Y.; Yan, Z.; Shen, W.; Shi, S.; Zhang, X.; Wang, H. Carboxymethylated-bacterial cellulose for copper and lead ion removal. J. Hazard. Mater. 2009, 161, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose-artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Rattanapanone, N. Synthesis and Characterization of Carboxymethyl Cellulose Powder and Films from Mimosa Pigra Peel. J. Appl. Polym. Sci. 2011, 122, 3218–3226. [Google Scholar] [CrossRef]
- Kirk, R.E.; Othmer, D.F. Cellulose Encyclopedia of Chemical Technology, 2nd ed.; Wiley: New York, NY, USA, 1967; Volume 4, pp. 593–683. [Google Scholar]
- Charpentier, D.; Mocanu, G.; Carpov, A.; Chapelle, S.; Merle, L.; Muller, G. New hydrophobically modified carboxymethyl cellulose derivatives. Carbohydr. Polym. 1997, 33, 177–186. [Google Scholar] [CrossRef]
- Adinugraha, M.P.; Marseno, D.W. Synthesis and characterization of sodium carboxymethylcellulose from Cavendish banana pseudo stem (Musa cavendishii LAMBERT). Carbohydr. Polym. 2005, 62, 164–169. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Eitssayeam, S.; Pengpat, K. Study of Carboxymethyl Cellulose from Papaya Peels Binder in Ceramics. Adv. Mater. Res. 2010, 93–94, 17–21. [Google Scholar] [CrossRef]
- El Ghzaoui, A.; Trompette, J.L.; Cassanas, G.; Bardet, L.; Fabregue, E. Comparative rheological behavior of some cellulosic ether derivatives. Langmuir 2001, 17, 1453–1456. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Luangkamin, S.; Tanprasert, K.; Suriyatem, R. Carboxymethyl cellulose film from durian rind. LWT-Food Sci. Technol. 2012, 48, 52–58. [Google Scholar] [CrossRef]
- Heinze, T.; Pfeiffer, K. Studies on the synthesis and characterization of carboxymethylcellulose. Angew. Makromol. Chem. 1999, 266, 37–45. [Google Scholar] [CrossRef]
- Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; Jantrawut, P.; Sommano, S.R.; et al. Synthesis, Characterization and Application of Carboxymethyl Cellulose from Asparagus officinalis Stalk End. Polymers 2021, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Waring, M.J.; Parsons, D. Physico-chemical characterization of carboxymethylated spun cellulose fibers. Biomaterials 2011, 22, 903–912. [Google Scholar] [CrossRef]
- Togrul, H.; Arslan, N. Production of carboxymethyl cellulose from sugar beet pulp cellulose and rheological behavior of carboxymethyl cellulose. Carbohydr. Polym. 2003, 54, 73–82. [Google Scholar] [CrossRef]
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y.Y. Optimisation of reaction condition for preparing carboxymethyl cellulose from sago waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Rachtanapun, P. Blended Films of Carboxymethyl Cellulose from Papaya Peel/Corn Starch Film Blends. Agric. Nat. Resour. 2009, 43, 259–266. [Google Scholar]
- Rachtanapun, P.; Kumthai, S.; Mulkarat, N.; Pintajam, N.; Suriyatem, R. Value added of mulberry paper waste by carboxymethylation for preparation a packaging film. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012081. [Google Scholar] [CrossRef] [Green Version]
- Rachtanapun, P.; Jantrawut, P.; Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; et al. Carboxymethyl Bacterial Cellulose from Nata de Coco: Effects of NaOH. Polymers 2021, 13, 348. [Google Scholar] [CrossRef]
- Kondo, T. The assignment of IR absorption bands due to free hydroxyl groups in cellulose. Cellulose 1997, 4, 60–63. [Google Scholar] [CrossRef]
- Fried, J.R. Polymer Science and Technology; Prentice-Hall: Saddle River, NJ, USA, 1995; p. 389. [Google Scholar]
- Bourtoom, T.; Chinnana, M.S. Preparation and properties of rice starch-chitosan blend biodegradable film. LWT Food Sci. Technol. 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Biocompatibility of bacterial cellulose based biomaterials. J. Funct. Biomater. 2012, 3, 864–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| MCA (g/15 g of Cellulose) | L* | a* | b* | ΔΕ | YI | WI |
|---|---|---|---|---|---|---|
| 6 | 67.99 a | 5.34 a | 14.65 a | 29.17 a | 30.81 a | 64.33 a |
| 12 | 75.22 b | 3.34 a, b | 15.10 a | 22.92 a | 28.68 a | 70.79 b |
| 18 | 77.57 a | 2.95 b | 15.85 a | 22.28 a | 29.58 a | 71.54 b |
| 24 | 78.00 b | 2.37 c | 17.28 a | 22.15 b | 31.67 a | 71.91 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Leksawasdi, N.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S.; et al. Effect of Monochloroacetic Acid on Properties of Carboxymethyl Bacterial Cellulose Powder and Film from Nata de Coco. Polymers 2021, 13, 488. https://doi.org/10.3390/polym13040488
Rachtanapun P, Klunklin W, Jantrawut P, Leksawasdi N, Jantanasakulwong K, Phimolsiripol Y, Seesuriyachan P, Chaiyaso T, Ruksiriwanich W, Phongthai S, et al. Effect of Monochloroacetic Acid on Properties of Carboxymethyl Bacterial Cellulose Powder and Film from Nata de Coco. Polymers. 2021; 13(4):488. https://doi.org/10.3390/polym13040488
Chicago/Turabian StyleRachtanapun, Pornchai, Warinporn Klunklin, Pensak Jantrawut, Noppol Leksawasdi, Kittisak Jantanasakulwong, Yuthana Phimolsiripol, Phisit Seesuriyachan, Thanongsak Chaiyaso, Warintorn Ruksiriwanich, Suphat Phongthai, and et al. 2021. "Effect of Monochloroacetic Acid on Properties of Carboxymethyl Bacterial Cellulose Powder and Film from Nata de Coco" Polymers 13, no. 4: 488. https://doi.org/10.3390/polym13040488
APA StyleRachtanapun, P., Klunklin, W., Jantrawut, P., Leksawasdi, N., Jantanasakulwong, K., Phimolsiripol, Y., Seesuriyachan, P., Chaiyaso, T., Ruksiriwanich, W., Phongthai, S., Sommano, S. R., Punyodom, W., Reungsang, A., & Ngo, T. M. P. (2021). Effect of Monochloroacetic Acid on Properties of Carboxymethyl Bacterial Cellulose Powder and Film from Nata de Coco. Polymers, 13(4), 488. https://doi.org/10.3390/polym13040488