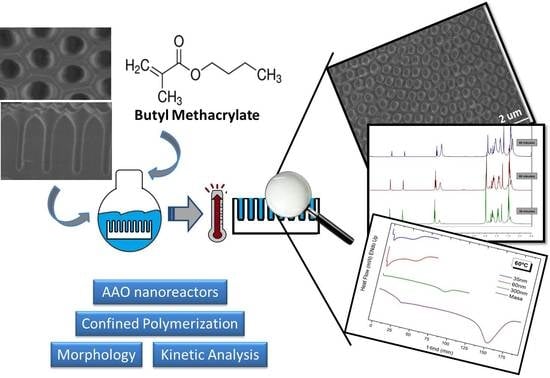
In Situ Synthesis of Poly(butyl methacrylate) in Anodic Aluminum Oxide Nanoreactors by Radical Polymerization: A Comparative Kinetics Analysis by Differential Scanning Calorimetry and 1H-NMR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AAO Templates Used as Nanoreactors
2.3. In Situ Polymerization of BMA in AAO Nanoreactors
2.4. Polymer Infiltration
2.5. Characterization Methods
3. Results
3.1. Free Radical Polymerization of BMA in AAO Nanoreactors
3.1.1. Kinetic Analysis by DSC
3.1.2. Kinetic Analysis by 1H-NMR
3.1.3. Polymer Nanostructures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, J.; Mijangos, C. Tailored polymer-based nanofibers and nanotubes by means of different infiltration methods into alumina nanopores. Langmuir 2009, 25, 1181–1187. [Google Scholar] [CrossRef]
- Cheng, W.; Steinhart, M.; Gösele, U.; Wehrspohn, R.B. Tree-like alumina nanopores generated in a non-steady-state anodization. J. Mater. Chem. 2007, 17, 3493–3495. [Google Scholar] [CrossRef]
- Sanz, B.; Blaszczyk-Lezak, I.; Mijangos, C.; Palacios, J.K.; Müller, A.J. New Double-Infiltration Methodology to Prepare PCL–PS Core–Shell Nanocylinders Inside Anodic Aluminum Oxide Templates. Langmuir 2016, 32, 7860–7865. [Google Scholar] [CrossRef] [PubMed]
- Rath, A.; Theato, P. Advanced AAO Templating of Nanostructured Stimuli-Responsive Polymers: Hype or Hope? Adv. Funct. Mater. 2019, 30, 1902959. [Google Scholar] [CrossRef]
- Blaszczyk-Lezak, I.; Desmaret, V.; Mijangos, C. Electrically conducting polymer nanostructures confined in anodized aluminum oxide templates (AAO). Express Polym. Lett. 2016, 10, 259–272. [Google Scholar] [CrossRef]
- Chen, J.-T.; Wei, T.-H.; Chang, C.-W.; Ko, H.-W.; Chu, C.-W.; Chi, M.-H.; Tsai, C.-C. Fabrication of polymer nanopeapods in the nanopores of anodic aluminum oxide templates using a double-solution wetting method. Macromolecules 2014, 47, 5227–5235. [Google Scholar] [CrossRef]
- Sha, Y.; Li, L.; Wang, X.; Wan, Y.; Yu, J.; Xue, G.; Zhou, D. Growth of Polymer Nanorods with Different Core–Shell Dynamics via Capillary Force in Nanopores. Macromolecules 2014, 47, 8722–8728. [Google Scholar] [CrossRef]
- Politidis, C.; Alexandris, S.; Sakellariou, G.; Steinhart, M.; Floudas, G. Dynamics of Entangled cis-1, 4-Polyisoprene Confined to Nanoporous Alumina. Macromolecules 2019, 52, 4185–4195. [Google Scholar] [CrossRef]
- Alexandris, S.; Papadopoulos, P.; Sakellariou, G.; Steinhart, M.; Butt, H.-J.; Floudas, G. Interfacial energy and glass temperature of polymers confined to nanoporous alumina. Macromolecules 2016, 49, 7400–7414. [Google Scholar] [CrossRef]
- Ok, S.; Steinhart, M.; Serbescu, A.; Franz, C.; Vaca Chávez, F.; Saalwächter, K. Confinement effects on chain dynamics and local chain order in entangled polymer melts. Macromolecules 2010, 43, 4429–4434. [Google Scholar] [CrossRef]
- Michell, R.M.; Müller, A.J. Confined crystallization of polymeric materials. Prog. Polym. Sci. 2016, 54–55, 183–213. [Google Scholar] [CrossRef]
- Safari, M.; Leon Boigues, L.; Shi, G.; Maiz, J.; Liu, G.; Wang, D.; Mijangos, C.; Müller, A.J. Effect of Nanoconfinement on the Isodimorphic Crystallization of Poly(butylene succinate-ran-caprolactone) Random Copolymers. Macromolecules 2020, 53, 6486–6497. [Google Scholar] [CrossRef]
- Müller, A.J.; Hu, W. Introduction to the “The effects of confinement on polymeric thermal transitions and nanostructuring” Special Volume. Prog. Polym. Sci. 2016, 100, 1–2. [Google Scholar]
- Michell, R.M.; Blaszczyk-Lezak, I.; Mijangos, C.; Müller, A.J. Confinement effects on polymer crystallization: From droplets to alumina nanopores. Polymer 2013, 54, 4059–4077. [Google Scholar] [CrossRef]
- Krutyeva, M.; Wischnewski, A.; Monkenbusch, M.; Willner, L.; Maiz, J.; Mijangos, C.; Arbe, A.; Colmenero, J.; Radulescu, A.; Holderer, O. Effect of nanoconfinement on polymer dynamics: Surface layers and interphases. Phys. Rev. Lett. 2013, 110, 108303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Simon, S.L. Equilibrium free-radical polymerization of methyl methacrylate under nanoconfinement. Polymer 2015, 66, 173–178. [Google Scholar] [CrossRef]
- Begum, F.; Zhao, H.; Simon, S.L. Modeling methyl methacrylate free radical polymerization: Reaction in hydrophilic nanopores. Polymer 2012, 53, 3238–3244. [Google Scholar] [CrossRef]
- Koh, Y.P.; Simon, S.L. Trimerization of monocyanate ester in nanopores. J. Phys. Chem. B 2010, 114, 7727–7734. [Google Scholar] [CrossRef]
- Uemura, T.; Ono, Y.; Kitagawa, K.; Kitagawa, S. Radical polymerization of vinyl monomers in porous coordination polymers: Nanochannel size effects on reactivity, molecular weight, and stereostructure. Macromolecules 2008, 41, 87–94. [Google Scholar] [CrossRef]
- Tarnacka, M.; Dulski, M.; Starzonek, S.; Adrjanowicz, K.; Mapesa, E.U.; Kaminski, K.; Paluch, M. Following kinetics and dynamics of DGEBA-aniline polymerization in nanoporous native alumina oxide membranes – FTIR and dielectric studies. Polymer 2015, 68, 253–261. [Google Scholar] [CrossRef]
- Tarnacka, M.; Dzienia, A.; Maksym, P.; Talik, A.; Zięba, A.; Bielas, R.; Kaminski, K.; Paluch, M. Highly Efficient ROP Polymerization of ε-Caprolactone Catalyzed by Nanoporous Alumina Membranes. How the Confinement Affects the Progress and Product of ROP Reaction. Macromolecules 2018, 51, 4588–4597. [Google Scholar] [CrossRef]
- Lee, L.-C.; Han, H.; Tsai, Y.-T.; Fan, G.-L.; Liu, H.-F.; Wu, C.-C.; Shyue, J.-J.; Sun, S.-S.; Liu, C.-L.; Chou, P.-T. Template-assisted in situ polymerization for forming blue organic light-emitting nanotubes. Chem. Commun. 2014, 50, 8208–8210. [Google Scholar] [CrossRef]
- Giussi, J.M.; Blaszczyk-Lezak, I.; Cortizo, M.S.; Mijangos, C. In-situ polymerization of styrene in AAO nanocavities. Polymer 2013, 54, 6886–6893. [Google Scholar] [CrossRef]
- Salsamendi, M.; Ballard, N.; Sanz, B.; Asua, J.M.; Mijangos, C. Polymerization kinetics of a fluorinated monomer under confinement in AAO nanocavities. RSC Adv. 2015, 5, 19220–19228. [Google Scholar] [CrossRef] [Green Version]
- Sanz, B.; Ballard, N.; Asua, J.M.; Mijangos, C. Effect of confinement on the synthesis of PMMA in AAO templates and modeling of free radical polymerization. Macromolecules 2017, 50, 811–821. [Google Scholar] [CrossRef]
- Zhao, H.; Simon, S.L. Methyl methacrylate polymerization in nanoporous confinement. Polymer 2011, 52, 4093–4098. [Google Scholar] [CrossRef]
- Tarnacka, M.; Madejczyk, O.; Dulski, M.; Wikarek, M.; Pawlus, S.; Adrjanowicz, K.; Kaminski, K.; Paluch, M. Kinetics and dynamics of the curing system. High pressure studies. Macromolecules 2014, 47, 4288–4297. [Google Scholar] [CrossRef]
- Sanz, B.; Ballard, N.; Marcos-Fernandez, A.; Asua, J.M.; Mijangos, C. Confinement effects in the step-growth polymerization within AAO templates and modeling. Polymer 2018, 140, 131–139. [Google Scholar] [CrossRef]
- Giussi, J.M.; Von Bilderling, C.; Alarcón, E.; Pietrasanta, L.I.; Hernandez, R.; Rafael, P.; Vázquez, M.; Mijangos, C.; Cortez, M.L.; Azzaroni, O. Thermo-responsive PNIPAm nanopillars displaying amplified responsiveness through the incorporation of nanoparticles. Nanoscale 2018, 10, 1189–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Victoria-Valenzuela, D.; Herrera-Ordonez, J.; Arcos-Casarrubias, A.; Vazquez-Torres, H. Kinetics of Bulk Free-Radical Polymerization of Butyl Methacrylate Isomers Studied by Reaction Calorimetry. Macromol. React. Eng. 2018, 12, 1700046. [Google Scholar] [CrossRef]
- Michailidis, M.; Verros, G.D.; Deliyanni, E.A.; Andriotis, E.G.; Achilias, D.S. An experimental and theoretical study of butyl methacrylate in situ radical polymerization kinetics in the presence of graphene oxide nanoadditive. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1433–1441. [Google Scholar] [CrossRef]
- Radzevicius, P.; Krivorotova, T.; Makuska, R. Synthesis by one-pot RAFT polymerization and properties of amphiphilic pentablock copolymers with repeating blocks of poly (2-hydroxyethyl methacrylate) and poly (butyl methacrylate). Eur. Polym. J. 2017, 87, 69–83. [Google Scholar] [CrossRef]
- Hiroshige, S.; Minato, H.; Nishizawa, Y.; Sasaki, Y.; Kureha, T.; Shibayama, M.; Uenishi, K.; Takata, T.; Suzuki, D. Temperature-dependent relationship between the structure and mechanical strength of volatile organic compound-free latex films prepared from poly (butyl acrylate-co-methyl methacrylate) microspheres. Polym. J. 2021, 53, 345–353. [Google Scholar] [CrossRef]
- Tian, Q.; Zhao, H.; Simon, S.L. Kinetic study of alkyl methacrylate polymerization in nanoporous confinement over a broad temperature range. Polymer 2020, 205, 122868. [Google Scholar] [CrossRef]
- Zhao, H.; Simon, S.L. Synthesis of polymers in nanoreactors: A tool for manipulating polymer properties. Polymer 2020, 211, 123112. [Google Scholar] [CrossRef]
- Maksym, P.; Tarnacka, M.; Wolnica, K.; Dzienia, A.; Erfurt, K.; Chrobok, A.; Zięba, A.; Bielas, R.; Kaminski, K.; Paluch, M. Studies on the hard confinement effect on the RAFT polymerization of a monomeric ionic liquid. Unexpected triggering of RAFT polymerization at 30° C. Polym. Chem. 2018, 9, 335–345. [Google Scholar] [CrossRef]
- Gorman, C.B.; Petrie, R.J.; Genzer, J. Effect of substrate geometry on polymer molecular weight and polydispersity during surface-initiated polymerization. Macromolecules 2008, 41, 4856–4865. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef]
- Mijangos, C.; Hernandez, R.; Martin, J. A review on the progress of polymer nanostructures with modulated morphologies and properties, using nanoporous AAO templates. Prog. Polym. Sci. 2016, 54, 148–182. [Google Scholar] [CrossRef]
- Sanz, B.; Von Bilderling, C.; Tuninetti, J.S.; Pietrasanta, L.; Mijangos, C.; Longo, G.S.; Azzaroni, O.; Giussi, J.M. Thermally-induced softening of PNIPAm-based nanopillar arrays. Soft Matter 2017, 13, 2453–2464. [Google Scholar] [CrossRef]
- Strehmel, V.; Laschewsky, A.; Wetzel, H.; Görnitz, E. Free radical polymerization of n-butyl methacrylate in ionic liquids. Macromolecules 2006, 39, 923–930. [Google Scholar] [CrossRef]
- León-Boigues, L.; von Bilderling, C.; Pietrasanta, L.; Azzaroni, O.; Mijangos, C.; Giussi, J.M. Reactivity Ratios and Surface Properties of Confined and Bulk ATRP Copolymerization of Butyl Methacrylate and 2-Hydroxyethyl Acrylate. ACS Appl. Polym. Mater. 2021, 3, 640–650. [Google Scholar]
- Odian, G. Principles of polymerization; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 0471274003. [Google Scholar]
- Zhao, H.Y.; Yu, Z.N.; Begum, F.; Hedden, R.C.; Simon, S.L. The effect of nanoconfinement on methyl methacrylate polymerization: Tg, molecular weight, and tacticity. Polymer 2014, 55, 4959–4965. [Google Scholar] [CrossRef]
- Richard, A. Interface and surface effects on the glass-transition temperature in thin polymer films. Faraday Discuss. 1994, 98, 219–230. [Google Scholar]
- Li, X.; King, T.A.; Pallikari-Viras, F. Characteristics of composites based on PMMA modified gel silica glasses. J. Non Cryst. Solids 1994, 170, 243–249. [Google Scholar] [CrossRef]
- Kalogeras, I.; Neagu, E. Interplay of surface and confinement effects on the molecular relaxation dynamics of nanoconfined poly (methyl methacrylate) chains. Eur. Phys. J. E 2004, 14, 193–204. [Google Scholar] [CrossRef]
- Blaszczyk-Lezak, I.; Hernaández, M.; Mijangos, C. One dimensional PMMA nanofibers from AAO templates. Evidence of confinement effects by dielectric and Raman analysis. Macromolecules 2013, 46, 4995–5002. [Google Scholar] [CrossRef]
- Maiz, J.; Martin, J.; Mijangos, C. Confinement Effects on the Crystallization of Poly (ethylene oxide) Nanotubes. Langmuir 2012, 28, 12296–12303. [Google Scholar] [CrossRef] [PubMed]
- Blaszczyk-Lezak, I.; Maiz, J.; Sacristán, J.; Mijangos, C. Monitoring the Thermal Elimination of Infiltrated Polymer from AAO Templates: An Exhaustive Characterization after Polymer Extraction. Ind. Eng. Chem. Res. 2011, 50, 10883–10888. [Google Scholar] [CrossRef]
- Li, L.; Zhou, D.; Huang, D.; Xue, G. Double glass transition temperatures of poly (methyl methacrylate) confined in alumina nanotube templates. Macromolecules 2014, 47, 297–303. [Google Scholar] [CrossRef]
- Zenasni, M.; Quintero-Jaime, A.; Benyoucef, A.; Benghalem, A. Synthesis and characterization of polymer/V2O5 composites based on poly (2-aminodiphenylamine). Polym. Compos. 2020, 1–11. [Google Scholar] [CrossRef]
- Usanmaz, A.; Ateş, J.; Doğan, A. Thermal and mechanical properties of microwave- and heat-cured poly(methyl methacrylate) used as dental base material. J. Appl. Polym. Sci. 2003, 90, 251–256. [Google Scholar] [CrossRef]
- Vargün, E.; Usanmaz, A. Polymerization of 2-hydroxyethyl acrylate in bulk and solution by chemical initiator and by ATRP method. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3957–3965. [Google Scholar]
- León-Boigues, L.; von Bilderling, C.; Pietrasanta, L.I.; Azzaroni, O.; Giussi, J.M.; Mijangos, C. A patterned butyl methacrylate-co-2-hydroxyethyl acrylate copolymer with softening surface and swelling capacity. Polymers 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappella, B.; Kaliappan, S.K.; Sturm, H. Using AFM force− distance curves to study the glass-to-rubber transition of amorphous polymers and their elastic− plastic properties as a function of temperature. Macromolecules 2005, 38, 1874–1881. [Google Scholar] [CrossRef]
- Cappella, B.; Silbernagl, D. Nanomechanical properties of polymer thin films measured by force–distance curves. Thin Solid Films 2008, 516, 1952–1960. [Google Scholar] [CrossRef]
- Cappella, B.; Kaliappan, S.K. Determination of thermomechanical properties of a model polymer blend. Macromolecules 2006, 39, 9243–9252. [Google Scholar] [CrossRef]







| AAO Pore Diameter (nm) | Electrolyte | Voltage (V) | Electrolyte Temperature (°C) | First Reaction Time (h) | Second Reaction Time | AAO Pore Length (µm) |
|---|---|---|---|---|---|---|
| 35 | 0.3 M H2C2O4 | 40 | 1–2 | 24 | 30 min | 1 |
| 72 h | 140 | |||||
| 140 | 2 wt% H3PO4; 0.02 M C6Al2O12 | 195 | 0.5–1 | 6 | 15 min | 1 |
| 24 h | 100 |
| Pore Diameter AAO (nm) | Temp. (°C) | ΔH (J/g) | ||
|---|---|---|---|---|
| 35 | 50 | * | 97 | 161 |
| 60 | * | 46 | 137 | |
| 60 | 50 | * | 145 | 163 |
| 60 | * | 57 | 174 | |
| 70 | * | 48 | 177 | |
| 300 | 60 | 12 | 107 | 179 |
| Bulk | 50 | 158 | 343 | 341 |
| 60 | 22 | 189 | 414 | |
| 70 | 8 | 94 | 392 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Boigues, L.; Pérez, L.A.; Mijangos, C. In Situ Synthesis of Poly(butyl methacrylate) in Anodic Aluminum Oxide Nanoreactors by Radical Polymerization: A Comparative Kinetics Analysis by Differential Scanning Calorimetry and 1H-NMR. Polymers 2021, 13, 602. https://doi.org/10.3390/polym13040602
León-Boigues L, Pérez LA, Mijangos C. In Situ Synthesis of Poly(butyl methacrylate) in Anodic Aluminum Oxide Nanoreactors by Radical Polymerization: A Comparative Kinetics Analysis by Differential Scanning Calorimetry and 1H-NMR. Polymers. 2021; 13(4):602. https://doi.org/10.3390/polym13040602
Chicago/Turabian StyleLeón-Boigues, Laia, Luis Andrés Pérez, and Carmen Mijangos. 2021. "In Situ Synthesis of Poly(butyl methacrylate) in Anodic Aluminum Oxide Nanoreactors by Radical Polymerization: A Comparative Kinetics Analysis by Differential Scanning Calorimetry and 1H-NMR" Polymers 13, no. 4: 602. https://doi.org/10.3390/polym13040602
APA StyleLeón-Boigues, L., Pérez, L. A., & Mijangos, C. (2021). In Situ Synthesis of Poly(butyl methacrylate) in Anodic Aluminum Oxide Nanoreactors by Radical Polymerization: A Comparative Kinetics Analysis by Differential Scanning Calorimetry and 1H-NMR. Polymers, 13(4), 602. https://doi.org/10.3390/polym13040602