Role of Electrospinning Parameters on Poly(Lactic-co-Glycolic Acid) and Poly(Caprolactone-co-Glycolic acid) Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Polymeric Solutions
2.3. Electrospinning
2.4. Scanning Electron Microscopy (SEM)
2.5. Characterization of the Polymeric Solutions
2.5.1. Density
2.5.2. Surface tension
2.5.3. Viscosity
2.5.4. Conductivity
3. Results and Discussion
3.1. Determination of the Electrospinning Parameters for PLGA and PCLGA
3.2. Characterization of the Solution Parameters: Density, Surface Tension, Viscosity and Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oprea, E.; Ficai, A.; Andronescu, E. Electrospun nanofibers for tissue engineering applications. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 77–95. [Google Scholar] [CrossRef]
- Liu, H.; Ding, X.; Zhou, G.; Li, P.; Wei, X.; Fan, Y. Electrospinning of Nanofibers for Tissue Engineering Applications. J. Nanomater. 2013, 2013, 495708. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Ghayour, H.; Ismail, A.F.; Nur, H.; Berto, F. Electrospun Nano-Fibers for Biomedical and Tissue Engineering Applications: A Comprehensive Review. Materials 2020, 13, 2153. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, H.; Ding, J. Fabrication of Electrospun Polymer Nanofibers with Diverse Morphologies. Molecules 2019, 24, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kai, D.; Liow, S.S.; Loh, X.J. Biodegradable polymers for electrospinning: Towards biomedical applications. Mater. Sci. Eng. C 2014, 45, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.-C.; Han, S.-J.; Shin, Y.-S.; Kang, I.-K. Fabrication of Biodegradable Polyester Nanocomposites by Electrospinning for Tissue Engineering. J. Nanomater. 2011, 2011, 929378. [Google Scholar] [CrossRef]
- Ranganathan, N.; Mugeshwaran, A.; Bensingh, R.J.; Kader, M.A.; Nayak, S.K. Biopolymeric Scaffolds for Tissue Engineering Application. In Biomedical Engineering and Its Applications in Healthcare; Springer: Singapore, 2019; pp. 249–274. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Sutera, F.; Gulino, E.F.; Morreale, M. Degradation and Recycling of Films Based on Biodegradable Polymers: A Short Review. Polymers 2019, 11, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Zhang, K.; Kai, D.; Li, Z.; Loh, X.J. Polyester elastomers for soft tissue engineering. Chem. Soc. Rev. 2018, 47, 4545–4580. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Baldursdottir, S.G.; Aho, J.; Qu, H.; Christensen, L.P.; Rantanen, J.; Yang, M. Electrospinnability of Poly Lactic-co-glycolic Acid (PLGA): The Role of Solvent Type and Solvent Composition. Pharm. Res. 2017, 34, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded nanofibers formed during electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Thompson, C.J.; Chase, G.G.; Yarin, A.L.; Reneker, D.H. Effects of parameters on nanofiber diameter determined from electrospinning model. Polymer 2007, 48, 6913–6922. [Google Scholar] [CrossRef]
- Robb, B.; Lennox, B. The electrospinning process, conditions and control. Electrospinning Tissue Regen. 2011, 51–66. [Google Scholar] [CrossRef]
- Amariei, N.; Manea, L.R.; Bertea, A.P.; Bertea, A.; Popa, A. The Influence of Polymer Solution on the Properties of Electrospun 3D Nanostructures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209. [Google Scholar] [CrossRef] [Green Version]
- Bera, B. Literature Review on Electrospinning Process (A Fascinating Fiber Fabrication Technique). Imp. J. Interdiscip. Res. IJIR 2016, 2, 972–984. [Google Scholar]
- Lee, D.; Kim, B.; Lee, S.; Lee, M.; Song, Y.; Lee, J. Titania nanofibers prepared by electrospinning. J. Korean Phys. Soc. 2006, 48, 1686–1690. [Google Scholar] [CrossRef]
- Angammana, C.J.; Jayaram, S.H. Analysis of the Effects of Solution Conductivity on Electrospinning Process and Fiber Morphology. IEEE Trans. Ind. Appl. 2011, 47, 1109–1117. [Google Scholar] [CrossRef]
- Moghe, A.K.; Hufenus, R.; Hudson, S.M.; Gupta, B.S. Effect of the addition of a fugitive salt on electrospinnability of poly(ε-caprolactone). Polymer 2009, 50, 3311–3318. [Google Scholar] [CrossRef]
- Lach, A.A.; Morris, H.L.; Martins, J.A.; Stace, E.T.; Carr, A.J.; Mouthuy, P.A. Pyridine as an additive to improve the deposition of continuous electrospun filaments. PLoS ONE 2019, 14, e0214419. [Google Scholar] [CrossRef] [Green Version]
- Herrero-herrero, M.; Gómez-tejedor, J.A.; Vallés-lluch, A. PLA/PCL electrospun membranes of tailored fi bres diameter as drug delivery systems. Eur. Polym. J. 2018, 99, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Jaworska, J.; Włodarczyk, J.; Karpeta-Jarząbek, P.; Janeczek, H.; Stojko, M.; Kasperczyk, J. Electrospun, drug-enriched bioresorbable nonwovens based on poly(glycolide-ɛ-caprolactone) and poly(D,L-lactide-glycolide) for urological applications. Polym. Degrad. Stab. 2019, 167, 94–101. [Google Scholar] [CrossRef]
- Jones, F.E.; Harris, G.L. ITS-90 density of water formulation for volumetric standards calibration. J. Res. Natl. Inst. Stand. Technol. 1992, 97, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Bini, T.B.; Gao, S.; Wang, S.; Ramakrishna, S. Poly(l-lactide-co-glycolide) biodegradable microfibers and electrospun nanofibers for nerve tissue engineering: An in vitro study. J. Mater. Sci. 2006, 41, 6453–6459. [Google Scholar] [CrossRef]
- Mehrasa, M.; Asadollahi, M.A.; Nasri-Nasrabadi, B.; Ghaedi, K.; Salehi, H.; Dolatshahi-Pirouz, A.; Arpanaei, A. Incorporation of mesoporous silica nanoparticles into random electrospun PLGA and PLGA/gelatin nanofibrous scaffolds enhances mechanical and cell proliferation properties. Mater. Sci. Eng. C 2016, 66, 25–32. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.H.; Yu, J.Y.; Zeng, H.M. Controlling numbers and sizes of beads in electrospun nanofibers. Polym. Int. 2008, 57, 632–636. [Google Scholar] [CrossRef]
- You, Y.; Min, B.M.; Lee, S.J.; Lee, T.S.; Park, W.H. In vitro degradation behavior of electrospun polyglycolide, polylactide, and poly(lactide-co-glycolide). J. Appl. Polym. Sci. 2005, 95, 193–200. [Google Scholar] [CrossRef]
- Luo, C.J.; Stride, E.; Edirisinghe, M. Mapping the influence of solubility and dielectric constant on electrospinning polycaprolactone solutions. Macromolecules 2012, 45, 4669–4680. [Google Scholar] [CrossRef]
- Uyar, T.; Besenbacher, F. Electrospinning of uniform polystyrene fibers: The effect of solvent conductivity. Polymer 2008, 49, 5336–5343. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, X.; Wu, L.; Han, Y.; Sheng, J. Study on morphology of electrospun poly(vinyl alcohol) mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- Pereira, I.H.; Ayres, E.; Averous, L.; Schlatter, G.; Hebraud, A.; Mendes, S.T.O.; Oréfice, R.L. Elaboration and characterization of coaxial electrospun poly(ε-caprolactone)/gelatin nanofibers for biomedical applications. Adv. Polym. Technol. 2014, 33, 1–10. [Google Scholar] [CrossRef]
- Petelska, A.D.; Kazimierska-Drobny, K.; Janicka, K.; Majewski, T.; Urbaniak, W. Understanding the unique role of phospholipids in the lubrication of natural joints: An interfacial tension study. Coatings 2019, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Somsap, J.; Kanjanapongkul, K.; Tepsorn, R. Effect of parameters on the morphology and fibre diameters of edible electrospun chitosan-cellulose acetate-gelatin hybrid nanofibres. MATEC Web Conf. 2018, 192. [Google Scholar] [CrossRef]
- Sohrabi, A.; Shaibani, P.M.; Thundat, T. The Effect of Applied Electric Field on the Diameter and Size Distribution of Electrospun Nylon6 Nanofibers. Scanning 2013, 35, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Wannatong, L.; Sirivat, A.; Supaphol, P. Effects of solvents on electrospun polymeric fibers: Preliminary study on polystyrene. Polym. Int. 2004, 53, 1851–1859. [Google Scholar] [CrossRef]
- Franck, A. Viscoelasticity and Dynamic Mechanical Testing; AN004; TA Instruments: New Castle, DE, USA, 1993. [Google Scholar]
- Mirtič, J.; Balažic, H.; Zupančič, Š.; Kristl, J. Effect of solution composition variables on electrospun alginate nanofibers: Response surface analysis. Polymers 2019, 11, 692. [Google Scholar] [CrossRef] [Green Version]
- Williams, G.R.; Raimi-Abraham, B.T.; Luo, C.J. Electrospinning fundamentals. In Nanofibres in Drug Delivery; UCL Press: London, UK, 2018; pp. 24–59. [Google Scholar] [CrossRef]





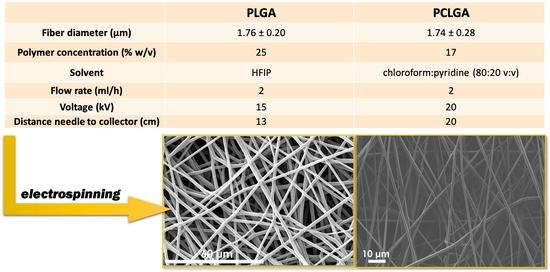
| PLGA | PCLGA | |
|---|---|---|
| Fiber diameter (µm) | 1.76 ± 0.20 | 1.74 ± 0.28 |
| Polymer concentration (% w/v) | 25 | 17 |
| Solvent | HFIP | chloroform:pyridine (80:20 v:v) |
| Flow rate (mL/h) | 2 | 2 |
| Voltage (kV) | 15 | 20 |
| Distance needle to collector (cm) | 13 | 20 |
| PLGA | PCLGA | |
|---|---|---|
| G′ (Pa) | 901.5 ± 1.6 | 1180.0 ± 90.4 |
| G″ (Pa) | 259.5 ± 6.5 | 232.4 ± 25.8 |
| tan (δ) | 0.288 ± 0.008 | 0.197 ± 0.020 |
| η′ (Pa·s) | 2.595 ± 0.065 | 2.324 ± 0.258 |
| η″ (Pa·s) | 9.015 ± 0.016 | 11.798 ± 0.904 |
| PLGA | PCLGA | |
|---|---|---|
| Density (g/mL) | 1.258 ± 0.010 | 1.018 ± 0.006 |
| Surface tension (mN/m) | 158.2 ± 0.5 | 136.8 ± 0.9 |
| Conductivity (S/cm) | 0.98 ± 0.01 | 0.31 ± 0.01 |
| Dynamic viscosity (Pa·s) | 2.595 ± 0.065 | 2.324 ± 0.256 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero-Herrero, M.; Gómez-Tejedor, J.A.; Vallés-Lluch, A. Role of Electrospinning Parameters on Poly(Lactic-co-Glycolic Acid) and Poly(Caprolactone-co-Glycolic acid) Membranes. Polymers 2021, 13, 695. https://doi.org/10.3390/polym13050695
Herrero-Herrero M, Gómez-Tejedor JA, Vallés-Lluch A. Role of Electrospinning Parameters on Poly(Lactic-co-Glycolic Acid) and Poly(Caprolactone-co-Glycolic acid) Membranes. Polymers. 2021; 13(5):695. https://doi.org/10.3390/polym13050695
Chicago/Turabian StyleHerrero-Herrero, María, José Antonio Gómez-Tejedor, and Ana Vallés-Lluch. 2021. "Role of Electrospinning Parameters on Poly(Lactic-co-Glycolic Acid) and Poly(Caprolactone-co-Glycolic acid) Membranes" Polymers 13, no. 5: 695. https://doi.org/10.3390/polym13050695
APA StyleHerrero-Herrero, M., Gómez-Tejedor, J. A., & Vallés-Lluch, A. (2021). Role of Electrospinning Parameters on Poly(Lactic-co-Glycolic Acid) and Poly(Caprolactone-co-Glycolic acid) Membranes. Polymers, 13(5), 695. https://doi.org/10.3390/polym13050695