New Polyporphyrin Arrays with Controlled Fluorescence Obtained by Diaxial Sn(IV)-Porphyrin Phenolates Chelation with Cu2+ Cation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Synthesis
3. Results and Discussion
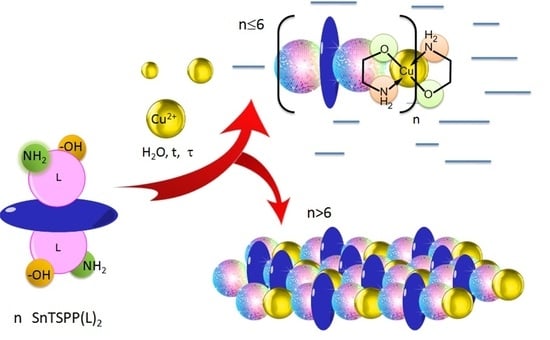
3.1. Synthesis and Structure
3.2. Thermogravimetric Analysis and Powder XRD
3.3. UV-Vis and IR-Spectral Studies
3.4. EPR Studies
3.5. NMR Spectroscopy Studies
3.6. Fluorescent Properties Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Chui, S.S. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Czaja, A.U.; Trukhan, N.; Müller, U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef]
- Henschel, A.; Gedrich, K.; Kraehnert, R.; Kaskel, S. Catalytic properties of MIL-101. Chem. Commun. 2008, 35, 4192–4194. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Y.; Ban, Y.; Jin, H.; Jiao, W.; Liu, X.; Yang, W. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science 2014, 346, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, P.; Ricco, R.; Doherty, C.M.; Liang, K.; Hill, A.J.; Styles, M.J. MOF positioning technology and device fabrication. Chem. Soc. Rev. 2014, 43, 5513–5560. [Google Scholar] [CrossRef]
- Li, Y.-N.; Wang, S.; Zhou, Y.; Bai, X.-J.; Song, G.-S.; Zhao, X.-Y.; Wang, T.-Q.; Qi, X.; Zhang, X.-M.; Fu, Y. Fabrication of Metal–Organic Framework and Infinite Coordination Polymer Nanosheets by the Spray Technique. Langmuir 2017, 33, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef]
- Ravon, U.; Domine, M.E.; Gaudillere, C.; Desmartin-Chomel, A.; Farrusseng, D. MOFs as acid catalysts with shape selectivity properties. New J. Chem. 2008, 32, 937–940. [Google Scholar] [CrossRef]
- Paille, G.; Gomez-Mingot, M.; Roch-Marchal, C.; Lassalle-Kaiser, B.; Mialane, P.; Fontecave, M.; Mellot-Draznieks, C.; Dolbecq, A. A Fully Noble Metal-Free Photosystem Based on Cobalt-Polyoxometalates Immobilized in a Porphyrinic Metal–Organic Framework for Water Oxidation. J. Am. Chem. Soc. 2018, 140, 3613–3618. [Google Scholar] [CrossRef]
- Kucheryavy, P.; Lahanas, N.; Lockard, J.V. Spectroscopic Evidence of Pore Geometry Effect on Axial Coordination of Guest Molecules in Metalloporphyrin-Based Metal Organic Frameworks. Inorg. Chem. 2018, 57, 3339–3347. [Google Scholar] [CrossRef]
- Pereira, C.F.; Figueira, F.; Mendes, R.F.; Rocha, J.; Hupp, J.T.; Farha, O.K.; Simões, M.M.Q.; Tomé, J.P.C.; Paz, F.A.A. Bifunctional Porphyrin-Based Nano-Metal–Organic Frameworks: Catalytic and Chemosensing Studies. Inorg. Chem. 2018, 57, 3855–3864. [Google Scholar] [CrossRef]
- Stassen, I.; Burtch, N.; Talin, A.; Falcaro, P.; Allendorf, M.; Ameloot, R. An updated roadmap for the integration of metal–organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 2017, 46, 3185–3241. [Google Scholar] [CrossRef]
- Gao, W.-Y.; Chrzanowski, M.; Ma, S. Metal–metalloporphyrin frameworks: A resurging class of functional materials. Chem. Soc. Rev. 2014, 43, 5841–5866. [Google Scholar] [CrossRef]
- Huh, S.; Kim, S.-J.; Kim, Y. Porphyrinic metal–organic frameworks from custom-designed porphyrins. CrystEngComm 2016, 18, 345–368. [Google Scholar] [CrossRef]
- Day, N.U.; Wamser, C.C.; Walter, M.G. Porphyrin polymers and organic frameworks. Polym. Int. 2015, 64, 833–857. [Google Scholar] [CrossRef]
- Ermakova, E.V.; Enakieva, Y.Y.; Meshkov, I.N.; Baranchikov, A.E.; Zvyagina, A.I.; Gorbunova, Y.G.; Tsivadze, A.Y.; Kalinina, M.A.; Arslanov, V.V. Bilayer Porphyrin-Graphene Templates for Self-Assembly of Metal-Organic Frameworks on the Surface. Macroheterocycles 2017, 10, 496–504. [Google Scholar] [CrossRef]
- Zvyagina, A.I.; Shiryaev, A.A.; Baranchikov, A.E.; Chernyshev, V.V.; Enakieva, Y.Y.; Raitman, O.A.; Ezhov, A.A.; Meshkov, I.N.; Grishanov, D.A.; Ivanova, O.S.; et al. Layer-by-layer assembly of porphyrin-based metal–organic frameworks on solids decorated with graphene oxide. New J. Chem. 2016, 41, 948–957. [Google Scholar] [CrossRef]
- Imai, H.; Misawa, K.; Munakata, H.; Uemori, Y. Water-soluble zinc porphyrins as artificial receptors for amino acids. Chem. Pharm. Bull. 2008, 56, 1470–1472. [Google Scholar] [CrossRef]
- Noworyta, K.; Kutner, W.; Wijesinghe, C.A.; Srour, S.G.; D’Souza, F. Nicotine, Cotinine, and Myosmine Determination Using Polymer Films of Tailor-Designed Zinc Porphyrins as Recognition Units for Piezoelectric Microgravimetry Chemosensors. Anal. Chem. 2012, 84, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Lee, C.-H.; Jeong, Y.-H.; Gee, H.-C.; Jang, W.-D. A zinc porphyrin-based molecular probe for the determination of contamination in commercial acetonitrile. Chem. Commun. 2012, 48, 5109–5111. [Google Scholar] [CrossRef]
- Gilday, L.C.; White, N.; Beer, P.D. Halogen- and hydrogen-bonding triazole-functionalised porphyrin-based receptors for anion recognition. Dalton Trans. 2013, 42, 15766. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Mamardashvili, G.M.; Kulikova, O.M.; Scheblykin, I.G.; Mamardashvili, N.Z.; Dehaen, W. Binding ability of first and second generation/carbazolylphenyl dendrimers with Zn(ii) tetraphenylporphyrin core towards small heterocyclic substrates. RSC Adv. 2014, 4, 19703–19709. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Mamardashvili, N.Z.; Koifman, O. Self-assembling systems based on porphirins. Russ. Chem. Rev. 2008, 77, 59–75. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Mamardashvili, N.Z. Self-organization of zinc(II) and tin(IV) porphyrinates into supramolecular trimers. Russ. J. Gen. Chem. 2013, 83, 1424–1428. [Google Scholar] [CrossRef]
- Sun, H.; Guo, K.; Gan, H.; Li, X.; Hunter, C.A. Influence of receptor flexibility on intramolecular H-bonding interactions. Org. Biomol. Chem. 2015, 13, 8053–8066. [Google Scholar] [CrossRef]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; John Wiley& Sons, Ltd: Chichester, UK, 2009; pp. 1–48. [Google Scholar]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, 1327. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Maltceva, O.V.; Lazovskiy, D.A.; Khodov, I.A.; Borovkov, V.; Mamardashvili, N.Z.; Koifman, O.I. Medium viscosity effect on fluorescent properties of Sn(IV)-tetra(4-sulfonatophenyl)porphyrin complexes in buffer solutions. J. Mol. Liq. 2019, 277, 1047–1053. [Google Scholar] [CrossRef]
- Herrmann, O.; Mehdi, S.H.; Corsini, A. Heterogeneous metal-insertion: A novel reaction with porphyrins. Can. J. Chem. 1978, 56, 1084–1087. [Google Scholar] [CrossRef]
- Bâtiu, C.; Jelic, C.; Leopold, N.; Cozar, O.; David, L. Spectroscopic investigations of new Cu(II), Co(II), Ni(II) complexes with γ-l-glutamyl amide as ligand. J. Mol. Struct. 2005, 744-747, 325–330. [Google Scholar] [CrossRef]
- Lewis, E.A.; Tolman, W.B. Reactivity of Dioxygen−Copper Systems. Chem. Rev. 2004, 104, 1047–1076. [Google Scholar] [CrossRef]
- Cabrele, C.; Langer, M.; Beck-Sickinger, A.G. Amino Acid Side Chain Attachment Approach and Its Application to the Synthesis of Tyrosine-Containing Cyclic Peptides. J. Org. Chem. 1999, 64, 4353–4361. [Google Scholar] [CrossRef]
- Rodante, F.; Marrosu, G.; Catalani, G. Thermal analysis of some α-amino acids with similar structures. Thermochim. Acta 1992, 194, 197–213. [Google Scholar] [CrossRef]
- Antina, E.V.; Balantseva, E.V.; Berezin, M.B. Oxidative degradation of porphyrins and metalloporphyrins under polythermal conditions. Russ. J. Gen. Chem. 2011, 81, 1222–1230. [Google Scholar] [CrossRef]
- Katoch, S.; Bajju, G.D.; Devi, G.; Ahmed, A. Synthesis, thermoanalytical and spectroscopic characterization of newly synthesized macrocyclic complexes of thallium(III) and tin(IV). J. Therm. Anal. Calorim. 2017, 130, 2157–2165. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, Q.; Zhao, D.; Zhang, W.; Wang, N.; Li, J. Synthesis and characterization of porphyrin-based porous coordination polymers obtained by supercritical CO2 extraction. J. Mater. Sci. 2018, 53, 10534–10542. [Google Scholar] [CrossRef]
- Rajalakshmi, V.; Vijayaraghavan, V.R.; Varghese, B.; Raghavan, A. Novel Michael Addition Products of Bis(amino acidato)metal(II) Complexes: Synthesis, Characterization, Dye Degradation, and Oxidation Properties. Inorg. Chem. 2008, 47, 5821–5830. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, O.; Tsujide, K.; Odani, A. Copper(II) complexes of tyrosine-containing dipeptides. Effects of side-chain groups on spectral and solution chemical properties and their structural implication. J. Am. Chem. Soc. 1985, 107, 659–666. [Google Scholar] [CrossRef]
- Sugimori, T.; Shibakawa, K.; Masuda, H.; Odani, A.; Yamauchi, O. Ternary metal(II) complexes with tyrosine-containing dipeptides. Structures of copper(II) and palladium(II) complexes involving L-tyrosylglycine and stabilization of copper(II) complexes due to intramolecular aromatic ring stacking. Inorg. Chem. 1993, 32, 4951–4959. [Google Scholar] [CrossRef]
- Nakamoto, K. (Ed.) Infrared and Raman Spectra of Inorganic and Coordination Compounds Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2009; 408p. [Google Scholar]
- Srivastava, K.P.; Singh, A. Facile Eco-friendly Synthesis, Spectral and Antimicrobial Activities of Copper—Amino Acid Complexes. IOSR J. Appl. Chem. 2016, 9, 1–6, e-ISSN 2278-5736. [Google Scholar] [CrossRef]
- Pogni, R.; Della Lunga, G.; Basosi, R. Multi-microwave frequency EPR in the structural characterization of copper(II) dipeptide complexes. J. Am. Chem. Soc. 1993, 115, 1546–1550. [Google Scholar] [CrossRef]
- Gala, L.; Lawson, M.; Jomova, K.; Zelenicky, L.; Congradyova, A.; Mazur, M.; Valko, M. EPR Spectroscopy of a Clinically Active (1:2) Copper(II)-Histidine Complex Used in the Treatment of Menkes Disease: A Fourier Transform Analysis of a Fluid CW-EPR Spectrum. Molecules 2014, 19, 980–991. [Google Scholar] [CrossRef]
- Mabbs, F.E.; Colisson, D. Electron Paramagnetic Resonance of d-Transition Metal Compounds; Elsevier: Amsterdam, The Netherlands, 1992; p. 102. [Google Scholar]
- Krinichnyi, V.I. 2-mm Wave Band EPR Spectroscopy of Condensed Systems; CRC Press: Boca Raton, FL, USA, 2018; pp. 33–62. [Google Scholar]
- Avezov, K.G.; Umarov, B.B.; Tursunov, M.A.; Parpiev, N.A.; Minin, V.V. Copper(II) complexes based on 2-thenoyltrifluoroacetone aroylhydrazones: Synthesis, spectroscopt and X-ray diffraction analysis. Russ. J. Coord. Chem. 2016, 42, 470–475. [Google Scholar] [CrossRef]
- Khodov, I.; Alper, G.; Mamardashvili, G.; Mamardashvili, N. Hybrid multi-porphyrin supramolecular assemblies: Synthesis and structure elucidation by 2D DOSY NMR studies. J. Mol. Struct. 2015, 1099, 174–180. [Google Scholar] [CrossRef]
- Watanabe, H.; Kamatani, Y.; Tamiaki, H. Coordination-Driven Dimerization of Zinc Chlorophyll Derivatives Possessing a Dialkylamino Group. Chem. Asian J. 2017, 12, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Efimov, S.V.; Zgadzay, Y.O.; Tarasova, N.B.; Klochkov, V.V. Evidence of oligomerization of bovine insulin in solution given by NMR. Eur. Biophys. J. 2018, 47, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, L.E.; Pavelyev, R.S.; Startseva, V.A.; Kiselev, S.V.; Galiullina, L.F.; Aganova, O.V.; Timerova, A.F.; Boichuk, S.V.; Azizova, Z.R.; Klochkov, V.V.; et al. Structural details on the interaction of biologically active sulfur-containing monoterpenoids with lipid membranes. J. Mol. Liq. 2020, 301, 112366. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Kaigorodova, E.Y.; Khodov, I.A.; Scheblykin, I.; Mamardashvili, N.Z.; Koifman, O.I.; Sheblykin, I. Micelles encapsulated Co(III)-tetra(4-sulfophenyl)porphyrin in aqueous CTAB solutions: Micelle formation, imidazole binding and redox Co(III)/Co(II) processes. J. Mol. Liq. 2019, 293, 111471. [Google Scholar] [CrossRef]
- Maltceva, O.; Mamardashvili, G.; Khodov, I.; Lazovskiy, D.; Khodova, V.; Krest’Yaninov, M.; Mamardashvili, N.; Dehaen, W. Molecular recognition of nitrogen-containing bases by Zn[5,15-bis-(2,6-dodecyloxyphenyl)]porphyrin. Supramol. Chem. 2017, 29, 360–369. [Google Scholar] [CrossRef]
- Zheng, G.; Stait-Gardner, T.; Kumar, P.A.; Torres, A.M.; Price, W.S. PGSTE-WATERGATE: An STE-based PGSE NMR sequence with excellent solvent suppression. J. Magn. Reson. 2008, 191, 159–163. [Google Scholar] [CrossRef]
- Oliva, A.I.; Gómez, K.; González, G.; Ballester, P. Diffusion-ordered spectroscopy (1H-DOSY) of Zn-porphyrin assemblies induced by coordination with DABCO. New J. Chem. 2008, 32, 2159–2163. [Google Scholar] [CrossRef]
- Timmerman, P.; Weidmann, J.-L.; Jolliffe, K.A.; Prins, L.J.; Reinhoudt, D.N.; Shinkai, S.; Frish, L.; Cohen, Y. NMR diffusion spectroscopy for the characterization of multicomponent hydrogen-bonded assemblies in solution. J. Chem. Soc. Perkin Trans. 2 2000, 2, 2077–2089. [Google Scholar] [CrossRef]
- Ksenofontov, A.A.; Stupikova, S.A.; Bocharov, P.S.; Lukanov, M.M.; Ksenofontova, K.V.; Khodov, I.A.; Antina, E.V. Novel fluorescent sensors based on zinc(II) bis(dipyrromethenate)s for furosemide detection in organic media. J. Photochem. Photobiol. A Chem. 2019, 382, 111899. [Google Scholar] [CrossRef]
- Holz, M.; Mao, X.; Seiferling, D.; Sacco, A. Experimental study of dynamic isotope effects in molecular liquids: Detection of translation-rotation coupling. J. Chem. Phys. 1996, 104, 669–679. [Google Scholar] [CrossRef]
- Waldeck, A.; Kuchel, P.W.; Lennon, A.J.; Chapman, B.E. NMR diffusion measurements to characterise membrane transport and solute binding. Prog. Nucl. Magn. Reson. Spectrosc. 1997, 30, 39–68. [Google Scholar] [CrossRef]
- Cabrita, E.J.; Berger, S. DOSY studies of hydrogen bond association: Tetramethylsilane as a reference compound for diffusion studies. Magn. Reson. Chem. 2001, 39, S142–S148. [Google Scholar] [CrossRef]
- Reddy, D.R.; Maiya, B.G. Bis(aryloxo) derivatives of tin(IV) porphyrins: Synthesis, spectroscopy and redox activity. J. Porphyrins Phthalocyanines 2002, 6, 3–11. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Chong, C.; Forsyth, C.; Langford, S.J.; Woodward, C.P. Investigations of rotamers in diaxial Sn(IV)porphyrin phenolates—towards a molecular timepiece. Tetrahedron 2008, 64, 8394–8401. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Lazovskiy, D.A.; Maltceva, O.V.; Mamardashvili, N.Z.; Koifman, O.I. The Sn(IV)-tetra(4-sulfonatophenyl) porphyrin complexes with antioxidants: Synthesis, structure, properties. Inorganica Chim. Acta 2019, 486, 468–475. [Google Scholar] [CrossRef]
- Lazovskiy, D.A.; Mamardashvili, G.M.; Khodov, I.A.; Mamardashvili, N.Z. Water soluble porphyrin-fluorescein triads: Design, DFT calculation and pH-change-triggered fluorescence response. J. Photochem. Photobiol. A Chem. 2020, 402, 112832. [Google Scholar] [CrossRef]













| Compounds | I | I-Cu-I | II | II-Cu-II |
|---|---|---|---|---|
| The maximum distance from the upper point of the ligand to the porphyrin core, Å | 7.061 | 10.39314 | 6.560 | 7.3955 |
| r(Sn-O), Å | 2.0517 | 2.0517 | 2.0517 | 1.9902 |
| r(Sn-N), Å | 4.238 | 4.1662 | 4.2244 | 4.1778 |
| r(Cu-O), Å | - | 1.81772 | - | 1.8220 |
| r(Cu-N), Å | - | 1.92909 | - | 1.9377 |
| <L-O-O-L(Ligand rotation angle) | 98° | 13° and 97° | 159° | 25° and 149° |
| <Sn-O-L (The bridge angle) | 122° | 145° | 131° | 172° |
| The angle between porphyrin end aromatic ligand planes | 41° | 41° 70° | 50 ° | 50° 87° |
| The angle between the porphyrin planesin the dimer | - | 9° | - | 9° |
| Compounds | Yield,% | Formula | Found/Calcd | |||
|---|---|---|---|---|---|---|
| Cu | C | H | N | |||
| I | - | C62H44N6O18S4Sn 1407.01 | - | 52.89 | 3.15 | 5.97 |
| II | - | C56H38N8O16S4Sn 1325.91 | - | 50.73 | 2.89 | 8.45 |
| I: Cu (1:1) | 94% | C62H44N6O18S4SnCu0.5 I-Cu-I 2877.57 | 2.19/ 2.21 | 51.67/ 51.72 | 3.06/ 3.08 | 5.81/ 5.84 |
| II: Cu (1:1) | 96% | C56H38N8O16S4SnCu0.5 II-Cu-II 2715.38 | 2.32/ 2.34 | 49.40/ 49.54 | 2.80/ 2.82 | 8.22/ 8.25 |
| I: Cu (1:5) a | 78% | C62H44N6O18S4SnCu1.17 Cu-[I-Cu]6 8892.89 | 4.98/ 5.00 | 50.48/ 50.24 | 2.96/ 2.99 | 5.64/ 5.67 |
| II: Cu (1:5) a | 84% | C56H38N8O16S4SnCu1.17 Cu-[II-Cu]6 8400.31 | 5.27/ 5.30 | 47.98/ 48.04 | 2.72/ 2.74 | 7.97/ 8.00 |
| I: Cu (1:5) b | 22% | C62H44N6O18S4SnCu [I-Cu]n n× [1471.56] | 4.27/ 4.32 | 50.62/ 50.60 | 3.00/ 3.014 | 5.68/ 5.71 |
| II: Cu (1:5) b | 16% | C56H38N8O16S4SnCu [II-Cu]n n× [1389.46] | 4.54/ 4.57 | 48.37/ 48.41 | 2.74/ 2.76 | 8.05/ 8.07 |
| Compound | Temperature Range (°C) | DTG Peak (°C) | TG Weight Loss (%) | Assignment | |
|---|---|---|---|---|---|
| Calcul. | Experim. | ||||
| II | 20–200 | 110 | 2.64 | 2.78 | uncoordinated water (2 mole) |
| 200–500 | 320.9 425.2 | 7.20 23.51 | 8.32 23.07 | dehydroxylation and deamination destruction of sulfo groups | |
| 500–800 | 690.1 820.9 | 22.35 10.88 | 20.80 12.02 | oxidation of the Ph-fragment of porphyrins oxidation of the Ph- fragment of ligands | |
| >900 | 33.41 | 33.01 | (SnC20H12N4O2 rest) | ||
| [II-Cu]n | 20–200 | 100 180 | 2.46 2.46 | 2.32 2.56 | uncoordinated water (2 mole) coordinated water (2 mole) |
| 200–500 | 352.9 425.2 | 6.71 21.91 | 7.23 18.47 | dehydroxylation and deamination destruction of sulfo groups | |
| 500–800 | 694.5 870.2 | 20.83 10.14 | 22.30 8.69 | oxidation of the Ph-fragment of porphyrins oxidation of the Ph- fragment of ligands | |
| >900 | 36.57 | 38.43 | SnC20H12N4O2, CuO rest | ||
| Compounds | UV-Vis Spectra, λnm(lgε) |
|---|---|
| I | 419 (5.04), 555 (4.06), 594 (3.57) |
| I-Cu-I | 418 (5.00), 554 (3.87), 595 (3.45), 610 (3.33) |
| Cu-[I-Cu]6 | 418 (4.98), 554 (3.78), 595 (3.40), 610 (3.89) |
| II | 419 (5.11), 554 (4.10), 593 (3.61) |
| II-Cu-II | 418 (5.05), 553 (4.07), 592 (3.48), 609 (3.29) |
| Cu-[II-Cu]6 | 418 (5.03), 553 (4.07), 592 (3.35), 609 (3.54) |
| I | Cu- [I-Cu]6 | I | Cu- [I-Cu]6 | II | Cu- [II-Cu]6 | II | Cu- [II-Cu]6 |
| NH3+ NH2 | COO- COO- | N-H N-H | O-H O-H | ||||
| 3188ν 3299ν 1655δd 1517δs 1246γr 1181γr | 3201ν 3230ν 1668δd 1534 δ 1200γ 1166γ | 1607νas 1384νs 646δas 580δs | 1660νas 1405νs 606δ 588δ | 3357ν 1619δ 764γw | 3430ν 1638δ 747γ | 3244ν 1378δd | - - |
C-O C-O | |||||||
| 1152ν | 1114ν | ||||||
| Cu-N Cu-N | Cu-O Cu-O | Cu-N Cu-N | Cu-O Cu-O | ||||
| - | 633ν | - | 472ν | - | 620ν | - | 480ν |
| Type of Protons | Chemical Shifts of Signals | Type of Protons | Chemical Shifts of Signals | ||||
|---|---|---|---|---|---|---|---|
| I | I-Cu-I | Cu[I-Cu]6 | II | II-Cu-II | Cu[II-Cu]6 | ||
| -COOH | 11.37 (s, 2H) | 11.35 (s, H) | - | -OH | 10.7 (s, 2H) | 10.6 (s, H) | - |
| -NH2 | 6.72 (s, 4H) | 6.71 (s, 2H), 6.91(brs, 2H) | 6.93 (brs, 2H) | -NH2 | 8.59 (s, 4H) | 8.79 (brs, 2H) | |
| -CH(L) | 4.37 (t, 2H) | 4.36 (t, H) 3.81 (t, H) | 3.78 (t, 2H) | -NH2 | 5.32 (s, 4H) | 5.35 (s, 4H) | 5.36 (s, 4H) |
| -CH2- | 3.19 (m, 4H) | 3.15 (m, 4H) | 3.11 (m, 4H) | Ph(L) | 5.97 (t, 2H) | 5.99(m, 2H) | 6.03 (t, 2H) |
| 2-Ph (L) | 5.51 (d, 4H) | 5.64 (m, 4H) | 5.82 (d, 4H) | Ph(L) | 2.92 (t, 2H) | 2.92 (t, 2H) | 2.91 (t, 2H) |
| 3-Ph (L) | 2.28 (d, 4H) | 2.30 (d, 4H) | 2.35 (d, 4H) | 2-Ph(Porph.) | 8.45 (d, 8H) | 8.46 (d, 8H) | 8.44 (d, 8H) |
| 2-Ph(Porph) | 8.36 (d, 8H) | 8.37 (d, 8H) | 8.38 (d, 8H) | 3-Ph(Porph.) | 8.25 (d, 8H) | 8.24(d, 8H) | 8.23 (d, 8H) |
| 3-Ph(Porph) | 8.14 (d, 8H) | 8.17 (d, 8H) | 8.15 (d, 8H) | β-Por | 9.10 (s, 8H) | 9.13 (s, 8H) | 9.12 (s, 8H) |
| β-Porph. | 9.41(s, 8H) | 9.43 (s, 8H) | 9.42 (s, 8H) | ||||
| I | I-Cu-I | I-[Cu-I]n |
|---|---|---|
| 2.96 | 2.26 | 1.55 |
| II | II-Cu-II | II-[Cu-II]n |
| 2.80 | 2.13 | 1.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamardashvili, G.M.; Lazovskiy, D.A.; Khodov, I.A.; Efimov, A.E.; Mamardashvili, N.Z. New Polyporphyrin Arrays with Controlled Fluorescence Obtained by Diaxial Sn(IV)-Porphyrin Phenolates Chelation with Cu2+ Cation. Polymers 2021, 13, 829. https://doi.org/10.3390/polym13050829
Mamardashvili GM, Lazovskiy DA, Khodov IA, Efimov AE, Mamardashvili NZ. New Polyporphyrin Arrays with Controlled Fluorescence Obtained by Diaxial Sn(IV)-Porphyrin Phenolates Chelation with Cu2+ Cation. Polymers. 2021; 13(5):829. https://doi.org/10.3390/polym13050829
Chicago/Turabian StyleMamardashvili, Galina M., Dmitriy A. Lazovskiy, Ilya A. Khodov, Artem E. Efimov, and Nugzar Z. Mamardashvili. 2021. "New Polyporphyrin Arrays with Controlled Fluorescence Obtained by Diaxial Sn(IV)-Porphyrin Phenolates Chelation with Cu2+ Cation" Polymers 13, no. 5: 829. https://doi.org/10.3390/polym13050829
APA StyleMamardashvili, G. M., Lazovskiy, D. A., Khodov, I. A., Efimov, A. E., & Mamardashvili, N. Z. (2021). New Polyporphyrin Arrays with Controlled Fluorescence Obtained by Diaxial Sn(IV)-Porphyrin Phenolates Chelation with Cu2+ Cation. Polymers, 13(5), 829. https://doi.org/10.3390/polym13050829