TD-DFT Simulation and Experimental Studies of a Mirrorless Lasing of Poly[(9,9-dioctylfluorenyl-2,7-diyl)-co-(1,4-diphenylene-vinylene-2-methoxy-5-{2-ethylhexyloxy}-benzene)]
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
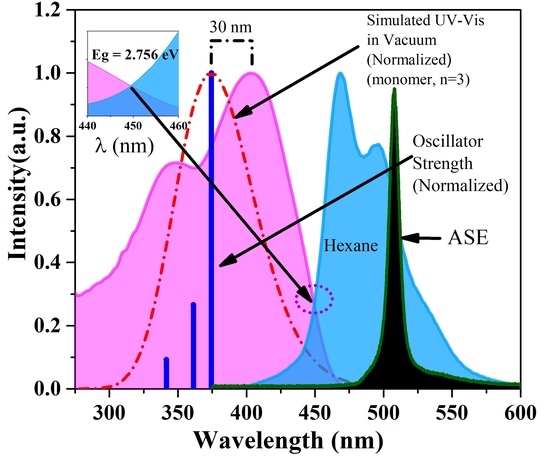
3.1. DFT Calculation
3.2. Absorption and Fluorescence Spectra of the CP in Toluene
3.3. ASE (Mirrorless Lasing) from CO PFO-co-PPV-MEHB in Toluene
4. Picosecond Time-Resolved Spectra of ASE in Toluene
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chénais, S.; Forget, S. Recent advances in solid-state organic lasers. Polym. Int. 2012, 61, 390–406. [Google Scholar] [CrossRef]
- Scherf, U.; Riechel, S.; Lemmer, U.; Mahrt, R.F. Conjugated polymers: Lasing and stimulated emission. Curr. Opin. Solid State Mater. Sci. 2001, 5, 143–154. [Google Scholar] [CrossRef]
- Mcgehee, M.; Heeger, A. Semiconducting (Conjugated) Polymers as Materials for Solid-State Lasers. Adv. Mater. 2000, 12, 1655–1668. [Google Scholar] [CrossRef]
- Ibnaouf, K.H. Photodynamic properties of poly [2-methoxy-5-(3′, 7′-dimethyloctyloxy)-1, 4-phenylenevinylene] under pulsed laser excitation. Opt. Laser Technol. 2020, 130, 106369. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Almotiri, A.R.; Prasad, S.; Aljaafreh, M.J.; Othman, A.H.S.; Masilamai, V. A temperature-tunable thiophene polymer laser. Polymers 2018, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- McNeill, C.R.; Greenham, N.C. Conjugated-polymer blends for optoelectronics. Adv. Mater. 2009, 38–39, 3840–3850. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, X. Oligomer Molecules for Efficient Organic Photovoltaics. Acc. Chem. Res. 2016, 49, 175–183. [Google Scholar] [CrossRef]
- Aljaafreh, M.J.; Prasad, S.; AlSalhi, M.S.; Alahmed, Z.A. Ultrafast dynamics of laser from green conjugated-oligomer in solution. Polymer 2019, 169, 106–114. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Bird, M.; Zhao, N. Charge transport physics of conjugated polymer field-effect transistors. Adv. Mater. 2010, 22, 3893–3898. [Google Scholar] [CrossRef]
- Brabec, C.J.; Dyakonov, V.; Parisi, J.; Sariciftci, N.S. Organic Photovoltaics: Concepts and Realization; Springer: New York, NY, USA, 2003. [Google Scholar]
- AlSalhi, M.S.; Alam, J.; Dass, L.A.; Raja, M. Recent advances in conjugated polymers for light emitting devices. Int. J. Mol. Sci. 2011, 12, 2036–2054. [Google Scholar] [CrossRef]
- Son, D.I.; Kim, H.H.; Cho, S.; Hwang, D.K.; Seo, J.W.; Choi, W.K. Carrier transport of inverted quantum dot LED with PEIE polymer. Org. Electron. Phys. Mater. Appl. 2014, 15, 886–892. [Google Scholar] [CrossRef]
- Prasad, S.; Ibnaouf, K.H.; Alsalhi, M.S.; Masilamani, V. Laser from the dimer state of a conjugated polymer (PFO) in solution. Polymer 2014, 55, 727–732. [Google Scholar] [CrossRef]
- Murphy, E. The semiconductor laser: Enabling optical communication. Nat. Photonics 2010, 4, 287. [Google Scholar] [CrossRef]
- Prasad, S.; Ibnaouf, K.H.; Alsalhi, M.S.; Devaraj, D.; Masilamani, V. High power amplified spontaneous emission from an oligomer in solution. J. Lumin. 2015, 168, 109–113. [Google Scholar] [CrossRef]
- Pisignano, D.; Anni, M.; Gigli, G.; Cingolani, R.; Zavelani-Rossi, M.; Lanzani, G.; Barbarella, G.; Favaretto, L. Amplified spontaneous emission and efficient tunable laser emission from a substituted thiophene-based oligomer. Appl. Phys. Lett. 2002, 81, 3534. [Google Scholar] [CrossRef]
- Aljaafreh, M.J.; AlSalhi, M.S.; Prasad, S. Design of tunable liquid laser based on presence of the conjugated-polymer counter influencing the spectral properties of the oligomer. Opt. Mater. 2021, 111, 110575. [Google Scholar] [CrossRef]
- Holzer, W.; Penzkofer, A.; Pertsch, T.; Danz, N.; Bräuer, A.; Kley, E.B.; Tillmann, H.; Bader, C.; Hörhold, H.H. Corrugated neat thin-film conjugated polymer distributed-feedback lasers. Appl. Phys. B Lasers Opt. 2002, 74, 333–342. [Google Scholar] [CrossRef]
- Bauer, C.; Giessen, H.; Schnabel, B.; Kley, E.B.; Schmitt, C.; Scherf, U.; Mahrt, R.F. A surface-emitting circular grating polymer laser. Adv. Mater. 2001, 13, 1161–1164. [Google Scholar] [CrossRef]
- Jory, M.J.; Barnes, W.L.; Samuel, I.D.W.; Turnbull, G.A.; Andrew, P. Relationship between photonic band structure and emission characteristics of a polymer distributed feedback laser. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 64, 1–6. [Google Scholar] [CrossRef]
- Moses, D. High quantum efficiency luminescence from a conducting polymer in solution: A novel polymer laser dye. Appl. Phys. Lett. 1992, 60, 3215–3216. [Google Scholar] [CrossRef]
- Brouwer, H.; Krasnikov, V.V.; Hilberer, A.; Wildeman, J.; Hadziioannou, G. Novel high efficiency copolymer laser dye in the blue wavelength region. Appl. Phys. Lett. 1995, 66, 3404–3406. [Google Scholar] [CrossRef]
- O’carroll, D.; Lieberwirth, I.; Redmond, G. Microcavity effects and optically pumped lasing in single conjugated polymer nanowires. Nat. Nanotechnol. 2007, 2, 180–184. [Google Scholar] [CrossRef]
- Mujamammi, W.M.; Prasad, S.; AlSalhi, M.S.; Masilamani, V. Relaxation oscillation with picosecond spikes in a conjugated polymer laser. Polymers 2016, 8, 364. [Google Scholar] [CrossRef]
- Hassan, M.U.; Liu, Y.C.; Butt, H.; Hasan, K.U.; Chang, J.F.; Olawoyin, A.A.; Friend, R.H. Low thresholds for a nonconventional polymer blend—Amplified spontaneous emission and lasing in F81-x:SYx system. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 15–21. [Google Scholar] [CrossRef]
- Oki, O.; Kushida, S.; Mikosch, A.; Hatanaka, K.; Takeda, Y.; Minakata, S.; Kuwabara, J.; Kanbara, T.; Dao, T.D.; Ishii, S.; et al. FRET-mediated near infrared whispering gallery modes: Studies on the relevance of intracavity energy transfer with Q-factors. Mater. Chem. Front. 2018, 2, 270–274. [Google Scholar] [CrossRef]
- Chilukuri, B.; Mazur, U.; Hipps, K.W. Structure, Properties, and Reactivity of Porphyrins on Surfaces and Nanostructures with Periodic DFT Calculations. Appl. Sci. 2020, 10, 740. [Google Scholar] [CrossRef]
- Han, D.; Li, J.; Zhang, Q.; He, Z.; Wu, Z.; Chu, J.; Lu, Y. Synthesis of π-Conjugated Polymers Containing Benzotriazole Units via Palladium-Catalyzed Direct CH Cross-Coupling Polycondensation for OLEDs Applications. Polymers 2021, 13, 254. [Google Scholar] [CrossRef]
- Aljaafreh, M.J.; Prasad, S.; AlSalhi, M.S.; Alahmed, Z.A.; Al-Mogren, M.M. Optically pumped intensive light amplification from a blue oligomer. Polymers 2019, 11, 1534. [Google Scholar] [CrossRef]
- Zou, X.; Wen, G.; Hu, R.; Dong, G.; Zhang, C.; Zhang, W.; Huang, H.; Dang, W. An Insight into the Excitation States of Small Molecular Semiconductor Y6. Molecules 2020, 25, 4118. [Google Scholar] [CrossRef] [PubMed]
- Reyes, Y.I.A.; Ting, L.-Y.; Tu, X.; Chen, H.-Y.T.; Chou, H.-H.; Coluccini, C. Mechanistic Studies of Hydrogen Evolution Reaction on Donor-Acceptor Conjugated Polymer Photocatalysts. Appl. Sci. 2020, 10, 7017. [Google Scholar] [CrossRef]
- Why Must I Have an emICCD? Available online: https://www.princetoninstruments.com/products/pi-max-family/pi-max/tech-notes/why-must-i-have-an-emiccd (accessed on 28 April 2021).
- Wang, Y.; Tsiminis, G.; Yang, Y.; Ruseckas, A.; Kanibolotsky, A.L.; Perepichka, I.F.; Skabara, P.J.; Turnbull, G.A.; Samuel, I.D.W. Broadly tunable deep blue laser based on a star-shaped oligofluorene truxene. Synth. Met. 2010. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 C.01. 2016. Available online: https://gaussian.com/relnotes/ (accessed on 28 April 2021).
- Chattopadhyaya, M.; Sen, S.; Alam, M.M.; Chakrabarti, S. The role of relativity and dispersion controlled inter-chain interaction on the band gap of thiophene, selenophene, and tellurophene oligomers. J. Chem. Phys. 2012, 136, 94904. [Google Scholar] [CrossRef]
- Peach, M.J.G.; Tellgren, E.I.; Sałek, P.; Helgaker, T.; Tozer, D.J. Structural and electronic properties of polyacetylene and polyyne from hybrid and coulomb-attenuated density functionals. J. Phys. Chem. A 2007, 111, 11930–11935. [Google Scholar] [CrossRef] [PubMed]
- Zade, S.S.; Bendikov, M. From oligomers to polymer: Convergence in the HOMO−LUMO gaps of conjugated oligomers. Org. Lett. 2006, 8, 5243–5246. [Google Scholar] [CrossRef]
- Franco, F.C., Jr.; Padama, A.A.B. DFT and TD-DFT study on the structural and optoelectronic characteristics of chemically modified donor-acceptor conjugated oligomers for organic polymer solar cells. Polymer 2016, 97, 55–62. [Google Scholar] [CrossRef]
- Dutta, T.; Woody, K.B.; Parkin, S.R.; Watson, M.D.; Gierschner, J. Conjugated polymers with large effective stokes shift: Benzobisdioxole-based poly (phenylene ethynylene)s. J. Am. Chem. Soc. 2009, 131, 17321–17327. [Google Scholar] [CrossRef]
- Keppler, S.; Sävert, A.; Körner, J.; Hornung, M.; Liebetrau, H.; Hein, J.; Kaluza, M.C. The generation of amplified spontaneous emission in high-power CPA laser systems. Laser Photon. Rev. 2016, 10, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhang, Y.; Chen, W.; George, T.F.; Li, S. Transient Aspects and Ultrafast Dynamical Processes of Amplified Spontaneous Emission in Conjugated Polymers. J. Phys. Chem. B 2018, 122, 10762–10766. [Google Scholar] [CrossRef]
- Jiang, Y.; Fang, M.; Chang, S.; Huang, J.; Chu, S.; Hu, S.; Liu, C.; Lai, W.; Huang, W. Towards Monodisperse Star-Shaped Ladder-Type Conjugated Systems: Design, Synthesis, Stabilized Blue Electroluminescence, and Amplified Spontaneous Emission. Chem. Eur. J. 2017, 23, 5448–5458. [Google Scholar] [CrossRef]
- Calzado, E.M.; Boj, P.G.; Díaz-García, M.A. Amplified spontaneous emission properties of semiconducting organic materials. Int. J. Mol. Sci. 2010, 11, 2546–2565. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljaafreh, M.J.; Prasad, S.; AlSalhi, M.S.; Alhandel, R.H.; Alsaigh, R.A. TD-DFT Simulation and Experimental Studies of a Mirrorless Lasing of Poly[(9,9-dioctylfluorenyl-2,7-diyl)-co-(1,4-diphenylene-vinylene-2-methoxy-5-{2-ethylhexyloxy}-benzene)]. Polymers 2021, 13, 1430. https://doi.org/10.3390/polym13091430
Aljaafreh MJ, Prasad S, AlSalhi MS, Alhandel RH, Alsaigh RA. TD-DFT Simulation and Experimental Studies of a Mirrorless Lasing of Poly[(9,9-dioctylfluorenyl-2,7-diyl)-co-(1,4-diphenylene-vinylene-2-methoxy-5-{2-ethylhexyloxy}-benzene)]. Polymers. 2021; 13(9):1430. https://doi.org/10.3390/polym13091430
Chicago/Turabian StyleAljaafreh, Mamduh J., Saradh Prasad, Mohamad S. AlSalhi, Raya H. Alhandel, and Reem A. Alsaigh. 2021. "TD-DFT Simulation and Experimental Studies of a Mirrorless Lasing of Poly[(9,9-dioctylfluorenyl-2,7-diyl)-co-(1,4-diphenylene-vinylene-2-methoxy-5-{2-ethylhexyloxy}-benzene)]" Polymers 13, no. 9: 1430. https://doi.org/10.3390/polym13091430
APA StyleAljaafreh, M. J., Prasad, S., AlSalhi, M. S., Alhandel, R. H., & Alsaigh, R. A. (2021). TD-DFT Simulation and Experimental Studies of a Mirrorless Lasing of Poly[(9,9-dioctylfluorenyl-2,7-diyl)-co-(1,4-diphenylene-vinylene-2-methoxy-5-{2-ethylhexyloxy}-benzene)]. Polymers, 13(9), 1430. https://doi.org/10.3390/polym13091430