Garcinol Encapsulated Ph-Sensitive Biodegradable Nanoparticles: A Novel Therapeutic Strategy for the Treatment of Inflammatory Bowel Disease
Abstract
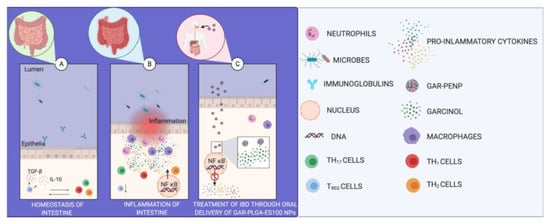
:1. Introduction
2. Materials
2.1. Preparation of GAR-PLGA-ES100 NPs
2.2. Characterization of GAR-PLGA-ES100 NPs
2.2.1. SEM
2.2.2. Size Distribution and Zeta Potential
2.2.3. Encapsulation Studies and Drug Loading Efficiency of GAR
2.2.4. In Vitro Drug Release Studies
2.3. Cell Culture Maintenance
2.4. In Vitro Localization of Coumarin-6 PLGA-ES100 NPs
2.5. In Vitro Cytotoxicity Analysis of GAR-PLGA-ES100 NPs in CACO-2 Cells
2.6. Lactate Dehydrogenase (LDH) Release Assay
2.7. Myeloperoxidase (MPO) Assay
2.8. Induction of Inflammation
2.9. Inhibition of Inflammation
2.10. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of GAR-PLGA-ES100 NPs
3.2. In Vitro Drug Release
3.3. Localization of the NPs in CACO-2 Cells
3.4. In Vitro Cytotoxicity Analysis
3.5. LDH Activity
3.6. MPO Activity
3.7. Induction and Inhibition of Inflammation Using Immunofluorescence Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lautenschläger, C.; Schmidt, C.; Lehr, C.-M.; Fischer, D.; Stallmach, A. PEG-Functionalized Microparticles Selectively Target Inflamed Mucosa in Inflammatory Bowel Disease. Eur. J. Pharm. Biopharm. 2013, 85, 578–586. [Google Scholar] [CrossRef]
- Zeeshan, M.; Ali, H.; Khan, S.; Mukhtar, M.; Khan, M.I.; Arshad, M. Glycyrrhizic Acid-Loaded PH-Sensitive Poly-(Lactic–co–Glycolic Acid) Nanoparticles for the Amelioration of Inflammatory Bowel Disease. Nanomedicine 2019, 14, 1945–1969. [Google Scholar] [CrossRef]
- Beloqui, A.; Coco, R.; Alhouayek, M.; Solinís, M.Á.; Rodríguez-Gascón, A.; Muccioli, G.G.; Préat, V. Budesonide-Loaded Nanostructured Lipid Carriers Reduce Inflammation in Murine DSS-Induced Colitis. Int. J. Pharm. 2013, 454, 775–783. [Google Scholar] [CrossRef]
- Qiao, H.; Fang, D.; Chen, J.; Sun, Y.; Kang, C.; Di, L.; Li, J.; Chen, Z.; Chen, J.; Gao, Y. Orally Delivered Polycurcumin Responsive to Bacterial Reduction for Targeted Therapy of Inflammatory Bowel Disease. Drug Deliv. 2017, 24, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Wachsmann, P.; Lamprecht, A. Polymeric Nanoparticles for the Selective Therapy of Inflammatory Bowel Disease. Methods Enzymol. 2012, 508, 377–397. [Google Scholar] [CrossRef]
- Hugot, J.-P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cézard, J.-P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M.; et al. Association of NOD2 Leucine-Rich Repeat Variants with Susceptibility to Crohn’s Disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef]
- Gazouli, M. NOD2/CARD15 Mediates the Inflammatory Responses in Inflammatory Bowel Diseases (IBDs). Ann. Gastroenterol. 2005, 18, 16–18. [Google Scholar]
- Fukata, M.; Shang, L.; Santaolalla, R.; Sotolongo, J.; Pastorini, C.; España, C.; Ungaro, R.; Harpaz, N.; Cooper, H.S.; Elson, G.; et al. Constitutive Activation of Epithelial TLR4 Augments Inflammatory Responses to Mucosal Injury and Drives Colitis-Associated Tumorigenesis. Inflamm. Bowel Dis. 2011, 17, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Kannan, N.; Guruvayoorappan, C. Protective Effect of Bauhinia Tomentosa on Acetic Acid Induced Ulcerative Colitis by Regulating Antioxidant and Inflammatory Mediators. Int. Immunopharmacol. 2013, 16, 57–66. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible Ginger-Derived Nanoparticles: A Novel Therapeutic Approach for the Prevention and Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, Y.; Hada, R.; Nakajima, H.; Fukuda, S.; Munakata, A. Improved Localizing Method of Radiopill in Measurement of Entire Gastrointestinal PH Profiles: Colonic Luminal PH in Normal Subjects and Patients with Crohn’s Disease. Am. J. Gastroenterol. 1997, 92, 114–118. [Google Scholar]
- Mohan, L.J.; Daly, J.S.; Ryan, B.M.; Ramtoola, Z. The Future of Nanomedicine in Optimising the Treatment of Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2019, 54, 18–26. [Google Scholar] [CrossRef]
- Yoshida, T.; Lai, T.C.; Kwon, G.S.; Sako, K. PH- and Ion-Sensitive Polymers for Drug Delivery. Expert Opin. Drug Deliv. 2013, 10, 1497–1513. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Naeem, M.; Bae, J.; Oshi, M.A.; Kim, M.-S.; Moon, H.R.; Lee, B.L.; Im, E.; Jung, Y.; Yoo, J.-W. Colon-Targeted Delivery of Cyclosporine A Using Dual-Functional Eudragit® FS30D/PLGA Nanoparticles Ameliorates Murine Experimental Colitis. Int. J. Nanomed. 2018, 13, 1225–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collnot, E.-M.; Ali, H.; Lehr, C.-M. Nano- and Microparticulate Drug Carriers for Targeting of the Inflamed Intestinal Mucosa. J. Control. Release 2012, 161, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Bhat, H.P.; Pai, R.J.; Boloor, R.; Palatty, P.L. The Chemistry and Medicinal Uses of the Underutilized Indian Fruit Tree Garcinia Indica Choisy (Kokum): A Review. Food Res. Int. 2011, 44, 1790–1799. [Google Scholar] [CrossRef]
- Tsai, M.-L.; Chiou, Y.-S.; Chiou, L.-Y.; Ho, C.-T.; Pan, M.-H. Garcinol Suppresses Inflammation-Associated Colon Carcinogenesis in Mice. Mol. Nutr. Food Res. 2014, 58, 1820–1829. [Google Scholar] [CrossRef] [PubMed]
- Gaonkar, R.H.; Ganguly, S.; Dewanjee, S.; Sinha, S.; Gupta, A.; Ganguly, S.; Chattopadhyay, D.; Debnath, M.C. Garcinol Loaded Vitamin E TPGS Emulsified PLGA Nanoparticles: Preparation, Physicochemical Characterization, In Vitro and In Vivo Studies. Sci. Rep. 2017, 7, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, M.-H.; Chang, W.-L.; Lin-Shiau, S.-Y.; Ho, C.-T.; Lin, J.-K. Induction of Apoptosis by Garcinol and Curcumin through Cytochrome c Release and Activation of Caspases in Human Leukemia HL-60 Cells. J. Agric. Food Chem. 2001, 49, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- El-Say, K.M.; El-Sawy, H.S. Polymeric Nanoparticles: Promising Platform for Drug Delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Barros, M.T. Polymeric Nanoparticles: A Study on the Preparation Variables and Characterization Methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Coco, R.; Memvanga, P.B.; Ucakar, B.; des Rieux, A.; Préat, V. PH-Sensitive Nanoparticles for Colonic Delivery of Curcumin in Inflammatory Bowel Disease. Int. J. Pharm. 2014, 473, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Hoa, L.T.M.; Chi, N.T.; Nguyen, L.H.; Chien, D.M. Preparation and Characterisation of Nanoparticles Containing Ketoprofen and Acrylic Polymers Prepared by Emulsion Solvent Evaporation Method. J. Exp. Nanosci. 2012, 7, 189–197. [Google Scholar] [CrossRef]
- Kumar, K.K.V.; Karnati, S.; Reddy, M.B.; Chandramouli, R. CACO-2 Cell Lines in Drug Discovery—An Updated Perspective. J. Basic Clin. Pharm. 2010, 1, 63–69. [Google Scholar] [PubMed]
- Tu, J.; Xu, Y.; Xu, J.; Ling, Y.; Cai, Y. Chitosan Nanoparticles Reduce LPS-Induced Inflammatory Reaction via Inhibition of NF-ΚB Pathway in Caco-2 Cells. Int. J. Biol. Macromol. 2016, 86, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Zhang, J.; Li, H.; Kortagere, S.; Sun, K.; Ding, L.; Ren, G.; Wang, Z.; Mani, S. Plant Flavonol Isorhamnetin Attenuates Chemically Induced Inflammatory Bowel Disease via a PXR-Dependent Pathway. J. Nutr. Biochem. 2014, 25, 923–933. [Google Scholar] [CrossRef] [Green Version]
- Dou, T.; Wang, J.; Han, C.; Shao, X.; Zhang, J.; Lu, W. Cellular Uptake and Transport Characteristics of Chitosan Modified Nanoparticles in Caco-2 Cell Monolayers. Int. J. Biol. Macromol. 2019, 138, 791–799. [Google Scholar] [CrossRef]
- Mastropietro, G.; Tiscornia, I.; Perelmuter, K.; Astrada, S.; Bollati-Fogolín, M. HT-29 and Caco-2 Reporter Cell Lines for Functional Studies of Nuclear Factor Kappa B Activation. Mediators Inflamm. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Ealia, S.A.M.; Saravanakumar, M.P. A Review on the Classification, Characterisation, Synthesis of Nanoparticles and Their Application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Mainardes, R.M.; Evangelista, R.C. PLGA Nanoparticles Containing Praziquantel: Effect of Formulation Variables on Size Distribution. Int. J. Pharm. 2005, 290, 137–144. [Google Scholar] [CrossRef]
- Coco, R.; Plapied, L.; Pourcelle, V.; Jérôme, C.; Brayden, D.J.; Schneider, Y.J.; Préat, V. Drug Delivery to Inflamed Colon by Nanoparticles: Comparison of Different Strategies. Int. J. Pharm. 2013, 440, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Liu, Z.; Kerdsakundee, N.; Zhang, M.; Zhang, F.; Liu, X.; Bauleth-Ramos, T.; Lian, W.; Mäkilä, E.; et al. Hierarchical Structured and Programmed Vehicles Deliver Drugs Locally to Inflamed Sites of Intestine. Biomaterials 2018, 185, 322–332. [Google Scholar] [CrossRef]
- Anwer, M.K.; Al-Shdefat, R.; Ezzeldin, E.; Alshahrani, S.M.; Alshetaili, A.S.; Iqbal, M. Preparation, Evaluation and Bioavailability Studies of Eudragit Coated PLGA Nanoparticles for Sustained Release of Eluxadoline for the Treatment of Irritable Bowel Syndrome. Front. Pharmacol. 2017, 8, 844. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.; Weigmann, B.; Neurath, M.F.; Collnot, E.M.; Windbergs, M.; Lehr, C.-M. Budesonide Loaded Nanoparticles with PH-Sensitive Coating for Improved Mucosal Targeting in Mouse Models of Inflammatory Bowel Diseases. J. Control. Release 2014, 183, 167–177. [Google Scholar] [CrossRef]
- Mongia, P.; Khatik, R.; Raj, R.; Jain, N.; Pathak, A.K. PH-Sensitive Eudragit S-100 Coated Chitosan Nanoparticles of 5-Amino Salicylic Acid for Colon Delivery. J. Biomater. Tissue Eng. 2014, 4, 738–743. [Google Scholar] [CrossRef]
- Makhlof, A.; Tozuka, Y.; Takeuchi, H. PH-Sensitive Nanospheres for Colon-Specific Drug Delivery in Experimentally Induced Colitis Rat Model. Eur. J. Pharm. Biopharm. 2009, 72, 1–8. [Google Scholar] [CrossRef]
- Pade, V.; Stavchansky, S. Estimation of the Relative Contribution of the Transcellular and Paracellular Pathway to the Transport of Passively Absorbed Drugs in the Caco-2 Cell Culture Model. Pharm. Res. 1997, 14, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Li, X.; Chen, D.; Le, C.; Zhu, C.; Hovgaard, L.; Yang, M. Novel Mucus-Penetrating Liposomes as a Potential Oral Drug Delivery System: Preparation, In Vitro Characterization, and Enhanced Cellular Uptake. Int. J. Nanomed. 2011, 3151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Kwon, S.J.; Sang, S.; Ju, J.; Zhou, J.; Ho, C.-T.; Huang, M.-T.; Yang, C.S. Effects of Garcinol and Its Derivatives on Intestinal Cell Growth: Inhibitory Effects and Autoxidation-Dependent Growth-Stimulatory Effects. Free Radic. Biol. Med. 2007, 42, 1211–1221. [Google Scholar] [CrossRef]
- Wang, T.; Li, J.; Shao, Y.; Yao, W.; Xia, J.; He, Q.; Huang, F. The Effect of Dietary Garcinol Supplementation on Oxidative Stability, Muscle Postmortem Glycolysis and Meat Quality in Pigs. Meat Sci. 2020, 161, 107998. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ho, P.C.-L.; Wong, F.C.; Sethi, G.; Wang, L.Z.; Goh, B.C. Garcinol: Current Status of Its Anti-Oxidative, Anti-Inflammatory and Anti-Cancer Effects. Cancer Lett. 2015, 362, 8–14. [Google Scholar] [CrossRef]
- Khan, A.; Alsahli, M.; Rahmani, A. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulli, B.; Ali, M.; Forghani, R.; Schob, S.; Hsieh, K.L.C.; Wojtkiewicz, G.; Linnoila, J.J.; Chen, J.W. Measuring Myeloperoxidase Activity in Biological Samples. PLoS ONE 2013, 8, e67976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Reis, S.B.; de Oliveira, C.C.; Acedo, S.C.; da Conceição Miranda, D.D.; Ribeiro, M.L.; Pedrazzoli, J.; Gambero, A. Attenuation of Colitis Injury in Rats Using Garcinia Cambogia Extract. Phyther. Res. 2009, 23, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.; Walz, G.; Singaram, C.; Lipman, M.L.; Zanker, B.; Muggia, A.; Antonioli, D.; Peppercorn, M.A.; Strom, T.B. Tumor Necrosis Factor-α, Interleukin-1β, and Interleukin-6 Expression in Inflammatory Bowel Disease. Dig. Dis. Sci. 1992, 37, 818–826. [Google Scholar] [CrossRef]
- O’Dwyer, A.M.; Lajczak, N.K.; Keyes, J.A.; Ward, J.B.; Greene, C.M.; Keely, S.J. Ursodeoxycholic Acid Inhibits TNFα-Induced IL-8 Release from Monocytes. Am. J. Physiol. Liver Physiol. 2016, 311, G334–G341. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Jia, Y.-Y.; Chen, S.-R.; Ye, J.-T.; Bu, X.-Z.; Hu, Y.; Ma, Y.-Z.; Guo, J.-L.; Liu, P.-Q. (E)-1-(4-Ethoxyphenyl)-3-(4-Nitrophenyl)-Prop-2-En-1-One Suppresses LPS-Induced Inflammatory Response through Inhibition of NF-ΚB Signaling Pathway. Int. Immunopharmacol. 2013, 15, 743–751. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Lawrence, L.; Mundkur, L. Novel Combinatorial Regimen of Garcinol and Curcuminoids for Non-Alcoholic Steatohepatitis (NASH) in Mice. Sci. Rep. 2020, 10, 7440. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Tuli, H.S.; Kaur, J.; Aggarwal, D.; Parashar, G.; Chaturvedi Parashar, N.; Kulkarni, S.; Kaur, G.; Sak, K.; Kumar, M.; et al. Garcinol Exhibits Anti-Neoplastic Effects by Targeting Diverse Oncogenic Factors in Tumor Cells. Biomedicines 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, F.H. Garcinol-Induced Apoptosis in Prostate and Pancreatic Cancer Cells Is Mediated by NF- KappaB Signaling. Front. Biosci. 2011, E3, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Protein of Interest | IL-6 | TNF- | LPS | IL-1 | Time |
|---|---|---|---|---|---|
| TNF- | 0.4 (μg/mL) | 0.9 (μg/mL) | 30 (μg/mL) | - | 3 h |
| NF-B | 0.2 (μg/mL) | 0.3 (μg/mL) | 20 (μg/mL) | - | 3 h |
| IL-8 | 0.4 (μg/mL) | 0.9 (μg/mL) | 30 (μg/mL) | 1 (μg/mL) | 45 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, E.M.; Borah, A.; Pillai, S.C.; Kumar, D.S. Garcinol Encapsulated Ph-Sensitive Biodegradable Nanoparticles: A Novel Therapeutic Strategy for the Treatment of Inflammatory Bowel Disease. Polymers 2021, 13, 862. https://doi.org/10.3390/polym13060862
Jacob EM, Borah A, Pillai SC, Kumar DS. Garcinol Encapsulated Ph-Sensitive Biodegradable Nanoparticles: A Novel Therapeutic Strategy for the Treatment of Inflammatory Bowel Disease. Polymers. 2021; 13(6):862. https://doi.org/10.3390/polym13060862
Chicago/Turabian StyleJacob, Eden Mariam, Ankita Borah, Sindhu C. Pillai, and D. Sakthi Kumar. 2021. "Garcinol Encapsulated Ph-Sensitive Biodegradable Nanoparticles: A Novel Therapeutic Strategy for the Treatment of Inflammatory Bowel Disease" Polymers 13, no. 6: 862. https://doi.org/10.3390/polym13060862
APA StyleJacob, E. M., Borah, A., Pillai, S. C., & Kumar, D. S. (2021). Garcinol Encapsulated Ph-Sensitive Biodegradable Nanoparticles: A Novel Therapeutic Strategy for the Treatment of Inflammatory Bowel Disease. Polymers, 13(6), 862. https://doi.org/10.3390/polym13060862