Optical Monitoring of Microplastics Filtrated from Wastewater Sludge and Suspended in Ethanol
Abstract
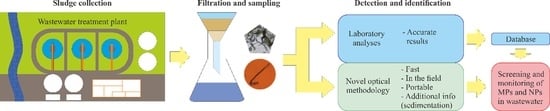
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Counting and Characterization of Microplastics with Raman Microscopy
2.3. Three Optics-Based Alternatives for a New Detection Method
2.4. Measurement Procedure with the Spectrophotometer and the New Optical Devices
3. Results and Discussion
3.1. Raman Microscopy
3.2. Specular Reflection Signal
3.3. Transmission Spectra
3.4. Sedimentation
3.5. Discussion of the Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alexy, P.; Anklam, E.; Emans, T.; Furfari, A.; Galgani, F.; Hanke, G.; Koelmans, A.; Pant, R.; Saveyn, H.; Sokull Kluettgen, B. Managing the Analytical Challenges Related to Micro- and Nanoplastics in the Environment and Food: Filling the Knowledge Gaps. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2020, 37, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Das, K.R.; Naik, M.M. Co-Selection of Multi-Antibiotic Resistance in Bacterial Pathogens in Metal and Microplastic Contaminated Environments: An Emerging Health Threat. Chemosphere 2019, 215, 846–857. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, H.; Fu, C.; Zhou, Y.; Dai, Z.; Li, Y.; Tu, C.; Luo, Y. The Distribution and Morphology of Microplastics in Coastal Soils Adjacent to the Bohai Sea and the Yellow Sea. Geoderma 2018, 322, 201–208. [Google Scholar] [CrossRef]
- Du, C.; Liang, H.; Li, Z.; Gong, J. Pollution Characteristics of Microplastics in Soils in Southeastern Suburbs of Baoding City, China. Int. J. Environ. Res. Public Health 2020, 17, 845. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kang, S.; Allen, S.; Allen, D.; Gao, T.; Sillanpää, M. Atmospheric Microplastics: A Review on Current Status and Perspectives. Earth-Science Rev. 2020, 203, 103118. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic Ingestion by Zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef] [PubMed]
- Bordos, G.; Urbanyi, B.; Micsinai, A.; Balazs, K.; Palotai, Z.; Szabo, I.; Hantosi, Z.; Szoboszlay, S. Identification of Microplastics in Fish Ponds and Natural Freshwater Environments of the Carpathian Basin, Europe. Chemosphere 2019, 216, 110–116. [Google Scholar] [CrossRef]
- Yu, Q.; Hu, X.; Yang, B.; Zhang, G.; Wang, J.; Ling, W. Distribution, Abundance and Risks of Microplastics in the Environment. Chemosphere 2020, 249, 126059. [Google Scholar] [CrossRef]
- Klein, S.; Dimzon, I.K.; Eubeler, J.; Knepper, T.P. Analysis, Occurrence, and Degradation of Microplastics in the Aqueous Environment. In Freshwater Microplastics: Emerging Environmental Contaminants? Wagner, M., Lambert, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 51–67. [Google Scholar] [CrossRef]
- Asamoah, B.O.; Uurasjärvi, E.; Räty, J.; Koistinen, A.; Roussey, M. Towards the Development of Portable and In Situ Optical Devices for Detection of Micro and Nanoplastics in Water: A Review on the Current Status. Polymers (Basel) 2021, 13, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Identification of Microplastic in Effluents of Waste Water Treatment Plants Using Focal Plane Array-Based Micro-Fourier-Transform Infrared Imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of Microplastics Using Raman Spectroscopy: Latest Developments and Future Prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.J. Microplastics in Wastewater Treatment Plants: Detection, Occurrence and Removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Garaba, S.P.; Dierssen, H.M. An Airborne Remote Sensing Case Study of Synthetic Hydrocarbon Detection Using Short Wave Infrared Absorption Features Identified from Marine-Harvested Macro- and Microplastics. Remote Sens. Environ. 2018, 205, 224–235. [Google Scholar] [CrossRef]
- Enfrin, M.; Dumée, L.F.; Lee, J. Nano/Microplastics in Water and Wastewater Treatment Processes–Origin, Impact and Potential Solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan-Reyes, S.; Gómez-Oliván, L.M.; Islas-Flores, H. COVID-19 in the Environment. Chemosphere 2021, 263, 127973. [Google Scholar] [CrossRef] [PubMed]
- Löder, M.G.J.; Imhof, H.K.; Ladehoff, M.; Löschel, L.A.; Lorenz, C.; Mintenig, S.; Piehl, S.; Primpke, S.; Schrank, I.; Laforsch, C.; et al. Enzymatic Purification of Microplastics in Environmental Samples. Environ. Sci. Technol. 2017, 51, 14283–14292. [Google Scholar] [CrossRef]
- Salmi, P.; Ryymin, K.; Karjalainen, A.K.; Mikola, A.; Uurasjärvi, E.; Talvitie, J. Particle Balance and Return Loops for Microplastics in a Tertiary-Level Wastewater Treatment Plant. Archive 2020. [Google Scholar] [CrossRef]
- Asamoah, B.O.; Kanyathare, B.; Roussey, M.; Peiponen, K.E. A Prototype of a Portable Optical Sensor for the Detection of Transparent and Translucent Microplastics in Freshwater. Chemosphere 2019, 231, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Asamoah, B.O.; Roussey, M.; Peiponen, K. On Optical Sensing of Surface Roughness of Flat and Curved Microplastics in Water. Chemosphere 2020, 254, 126789. [Google Scholar] [CrossRef]
- Asamoah, B.; Amoani, J.; Roussey, M.; Peiponen, K.-E. Laser Beam Scattering for the Detection of Flat, Curved, Smooth, and Rough Microplastics in Water. Opt. Rev. 2020, 27, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Asamoah, B.O.; Kanyathare, B.; Peiponen, K.-E. Table Model and Portable Optical Sensors for the Monitoring of Time-Dependent Liquid Spreading over Rough Surfaces. J. Eur. Opt. Soc. 2018, 14. [Google Scholar] [CrossRef] [Green Version]
- Erf, R.K. Speckle Metrology; Erf, R.K., Ed.; Academic Press: Cambridge, MA, USA, 1978; Vol. 1, ISBN 0-12-241360-1. [Google Scholar] [CrossRef]
- Fujii, H.; Asakura, T.; Shindo, Y. Measurements of Surface Roughness Properties by Means of Laser Speckle Techniques. Opt. Commun. 1976, 16. [Google Scholar] [CrossRef]
- Kim, J.W.; Jang, H.; Kim, G.H.; Jun, S.W.; Kim, C.-S. Multi-Spectral Laser Speckle Contrast Images Using a Wavelength-Swept Laser. J. Biomed. Opt. 2019, 24, 1. [Google Scholar] [CrossRef]
- Peiponen, K.E.; Räty, J.; Ishaq, U.; Pélisset, S.; Ali, R. Outlook on Optical Identification of Micro- and Nanoplastics in Aquatic Environments. Chemosphere 2019, 214, 424–429. [Google Scholar] [CrossRef]
- Kanyathare, B.; Asamoah, B.O.; Ishaq, U.; Amoani, J. Optical Transmission Spectra Study in Visible and Near-Infrared Spectral Range for Identification of Rough Transparent Plastics in Aquatic Environments. Chemosphere 2020, 248, 126071. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Guardado, A.; Money, M.; McKinney, N.; Chanda, D. Multi-Spectral Infrared Spectroscopy for Robust Plastic Identification. Appl. Opt. 2015, 54, 7396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; Li, J. The Removal of Microplastics in the Wastewater Treatment Process and Their Potential Impact on Anaerobic Digestion Due to Pollutants Association. Chemosphere 2020, 251, 126360. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.S.; Sun, L.; Huang, X.; Zhou, J.Z. Evaporation of Ethanol/Water Mixture Droplets on a Pillar-like PDMS Surface. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 574, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Huth-Fehre, T.; Feldhoff, R.; Kantimm, T.; Quick, L.; Winter, F.; Cammann, K.; van den Broek, W.; Wienke, D.; Melssen, W.; Buydens, L. NIR-Remote Sensing and Artificial Neural Networks for Rapid Identification of Post Consumer Plastics. J. Mol. Struct. 1995, 348, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Durbin, S.D.; Arakelian, S.M.; Shen, Y.R. Laser-Induced Diffraction Rings from a Nematic- Liquid-Crystal Film. Opt. Lett. 1981, 6, 411–413. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, S.J.; Jeon, S.-W.; Ju, S.; Park, C.-S.; Han, W.-T.; Lee, B.H. Thermo-Optic Coefficient Measurement of Liquids Based on Simultaneous Temperature and Refractive Index Sensing Capability of a Two-Mode Fiber Interferometric Probe. Opt. Express 2012, 20, 23744. [Google Scholar] [CrossRef]
- Arnaud, N.; Georges, J. Investigation of the Thermal Lens Effect in Water-Ethanol Mixtures: Composition Dependence of the Refractive Index Gradient, the Enhancement Factor and the Soret Effect. Spectrochim. Acta-Part. A Mol. Biomol. Spectrosc. 2001, 57, 1295–1301. [Google Scholar] [CrossRef]
- Shen, J.; Jia, X. Diffraction of a Plane Wave by an Infinitely Long Circular Cylinder or a Sphere: Solution from Mie Theory. Appl. Opt. 2013, 52, 5707–5712. [Google Scholar] [CrossRef]
- Tavassoly, M.T.; Hosseini, S.R.; Fard, A.M.; Naraghi, R.R. Applications of Fresnel Diffraction from the Edge of a Transparent Plate in Transmission. Appl. Opt. 2012, 51, 7170. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, A.; White, J.R. Densification of Polystyrene under Ultraviolet Irradiation. J. Mater. Sci. Lett. 2001, 20, 2089–2090. [Google Scholar] [CrossRef]
- Iacopi, A.V.; White, J.R. Residual Stress, Aging, and Fatigue Fracture in Injection Molded Glassy Polymers, I. Polystyrene. J. Appl. Polym. Sci. 1987, 33, 577–606. [Google Scholar] [CrossRef]
- Chicea, D. Speckle Size, Intensity and Contrast Measurement Application in Micron-Size Particle Concentration Assessment. Eur. Phys. J. Appl. Phys. 2007, 40, 305–310. [Google Scholar] [CrossRef]





| Step | Reagent | Incubation Temperature and Time |
|---|---|---|
| 1 | Hydrogen peroxide 30% | 60 °C, 1 d |
| 2 | Protease in Tris-HCl buffer (1:5, pH = 9) | 50 °C, 1 d |
| 3 | Lipase in Tris-HCl buffer (1:20, pH = 9) | 40 °C, 1 d |
| 4 | Cellulase in NaOAc buffer (1:4, pH = 5) | 40 C, 3 × 1 d |
| 5 | Hydrogen peroxide 30% | 60 C, 1 d |
| 6 | Chitinase in NaOAc (1:20, pH = 5) | 37 °C, 1 d |
| 7 | Hydrogen peroxide 30% | 60 °C, 1 d |
| 8 | Density separation with ZnCl2 (ρ = 1.6–1.8 g.cm−3) | Room temperature, 1–3 d |
| Sample A | Sample B | Sample C | |||||
|---|---|---|---|---|---|---|---|
| Dry Weight [g] | 11.5 | 13.8 | 13.9 | ||||
| Proportion of DW analyzed with Raman [%] | 0.9 | 0.9 | 2 | ||||
| Fibers counted | 6 | 4 | 15 | ||||
| Particles counted | 8 | 7 | 6 | ||||
| MPs g−1 DW | 130 | 85 | 85 | ||||
| MPs/mL | 19 | 15 | 17 | ||||
| MP types | F | P | F | P | F | P | Density in pristine form [g cm−3] |
| PP | 0 | 3 | 0 | 1 | 0 | 3 | 0.85 |
| PE | 1 | 0 | 0 | 1 | 3 | 0 | 0.94 |
| PET | 5 | 1 | 4 | 2 | 12 | 1 | 1.38 |
| PS | 0 | 1 | 0 | 0 | 0 | 1 | 1.05 |
| POM | 0 | 3 | 0 | 3 | 0 | 1 | 1.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asamoah, B.O.; Salmi, P.; Räty, J.; Ryymin, K.; Talvitie, J.; Karjalainen, A.K.; Kukkonen, J.V.K.; Roussey, M.; Peiponen, K.-E. Optical Monitoring of Microplastics Filtrated from Wastewater Sludge and Suspended in Ethanol. Polymers 2021, 13, 871. https://doi.org/10.3390/polym13060871
Asamoah BO, Salmi P, Räty J, Ryymin K, Talvitie J, Karjalainen AK, Kukkonen JVK, Roussey M, Peiponen K-E. Optical Monitoring of Microplastics Filtrated from Wastewater Sludge and Suspended in Ethanol. Polymers. 2021; 13(6):871. https://doi.org/10.3390/polym13060871
Chicago/Turabian StyleAsamoah, Benjamin O., Pauliina Salmi, Jukka Räty, Kalle Ryymin, Julia Talvitie, Anna K. Karjalainen, Jussi V. K. Kukkonen, Matthieu Roussey, and Kai-Erik Peiponen. 2021. "Optical Monitoring of Microplastics Filtrated from Wastewater Sludge and Suspended in Ethanol" Polymers 13, no. 6: 871. https://doi.org/10.3390/polym13060871
APA StyleAsamoah, B. O., Salmi, P., Räty, J., Ryymin, K., Talvitie, J., Karjalainen, A. K., Kukkonen, J. V. K., Roussey, M., & Peiponen, K. -E. (2021). Optical Monitoring of Microplastics Filtrated from Wastewater Sludge and Suspended in Ethanol. Polymers, 13(6), 871. https://doi.org/10.3390/polym13060871