Biofilm Formation on the Surface of (Poly)Ether-Ether-Ketone and In Vitro Antimicrobial Efficacy of Photodynamic Therapy on Peri-Implant Mucositis
Abstract
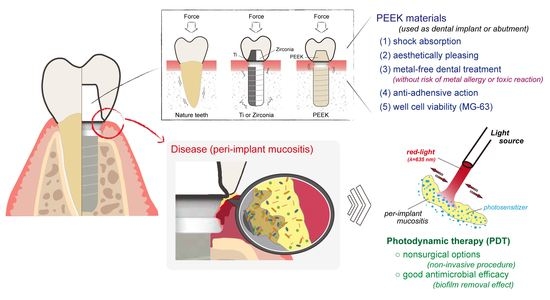
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Surface Treatment
2.2. Surface Roughness and Morphologies
2.3. Hydrophilicity
2.4. Microbial Cultures
2.5. Biofilm Formation Assay
2.6. Biofilm Removal Assay
2.7. Cell Viability Assay
2.8. Statistical Analysis
3. Results
3.1. Morphologies, Topography, and Surface Roughness
3.2. Hydrophilicity (Contact Angle)
3.3. Biofilm Formation Ability
3.4. Biofilm Removal Efficacy
3.5. Cell Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zotti, F.; Pappalardo, D.; Capocasale, G.; Sboarina, A.; Bertossi, D.; Albanese, M. Aesthetic dentistry, how you say and how you see: A 500-people survey on digital preview and color perception. Clin. Cosmet. Investig. Dent. 2020, 12, 377–389. [Google Scholar] [CrossRef]
- Puig, C.P. A retrospective study of edentulous patients rehabilitated according to the ‘all-on-four’or the ‘all-on-six’immediate function concept using flapless computer-guided implant surgery. Eur. J. Oral Implant. 2010, 3, 155–163. [Google Scholar]
- Nogueira, T.E.; Dias, D.R.; Leles, C.R. Mandibular complete denture versus single-implant overdenture: A systematic review of patient-reported outcomes. J. Oral. Rehabil. 2017, 44, 1004–1016. [Google Scholar] [CrossRef]
- Horita, S.; Sugiura, T.; Yamamoto, K.; Murakami, K.; Imai, Y.; Kirita, T. Biomechanical analysis of immediately loaded implants according to the “All-on-Four” concept. J. Prosthodont. Res. 2017, 61, 123–132. [Google Scholar] [CrossRef]
- Steinemann, S.G. Titanium—The material of choice? Periodontology 2000 1998, 17, 7–21. [Google Scholar] [CrossRef]
- Wandiyanto, J.V.; Truong, V.K.; Al Kobaisi, M.; Juodkazis, S.; Thissen, H.; Bazaka, O.; Bazaka, K.; Crawford, R.J.; Ivanova, E.P. The fate of osteoblast-like MG-63 cells on pre-infected bactericidal nanostructured titanium surfaces. Materials 2019, 12, 1575. [Google Scholar] [CrossRef] [Green Version]
- Azizi, B.; Budimir, A.; Bago, I.; Mehmeti, B.; Jakovljević, S.; Kelmendi, J.; Stanko, A.P.; Gabrić, D. Antimicrobial efficacy of photodynamic therapy and light-activated disinfection on contaminated zirconia implants: An in vitro study. Photodiagnosis Photodyn. Ther. 2018, 21, 328–333. [Google Scholar] [CrossRef]
- Yan, H.; Afroz, S.; Dalanon, J.; Goto, N.; Hosoki, M.; Matsuka, Y. Metal allergy patient treated by titanium implant denture: A case report with at least 4-year follow-up. Clin. Case. Rep. 2018, 6, 1972–1977. [Google Scholar] [CrossRef]
- Přikrylová, J.; Procházková, J.; Podzimek, Š. Side effects of dental metal implants: Impact on human health (metal as a risk factor of implantologic treatment). Biomed. Res. Int. 2019, 2019, 2519205. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 98, 1294–1305. [Google Scholar] [CrossRef]
- Martins, R.; Cestari, T.M.; Arantes, R.V.N.; Santos, P.S.; Taga, R.; Carbonari, M.J.; Oliveira, R.C. Osseointegration of zirconia and titanium implants in a rabbit tibiae model evaluated by microtomography, histomorphometry and fluorochrome labeling analyses. J. Periodont. Res. 2018, 53, 210–221. [Google Scholar] [CrossRef]
- Soon, G.; Pingguan-Murphy, B.; Lai, K.W.; Akbar, S.A. Review of zirconia-based bioceramic: Surface modification and cellular response. Ceram. Int. 2016, 42, 12543–12555. [Google Scholar] [CrossRef]
- Kondo, T.; Komine, F.; Honda, J.; Takata, H.; Moriya, Y. Effect of veneering materials on fracture loads of implant-supported zirconia molar fixed dental prostheses. J. Prosthodont. Res. 2018, 63, 140–144. [Google Scholar] [CrossRef]
- Gama, L.T.; Duque, T.M.; Özcan, M.; Philippi, A.G.; Mezzomo, L.A.M.; Gonçalves, T.M.S.V. Adhesion to high-performance polymers applied in dentistry: A systematic review. Dent. Mater. 2020, 36, e93–e108. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Karthik, N.; Xiong, D.; Liu, Y. Bio-inspired surface modification of PEEK through the dual cross-linked hydrogel layers. J. Mech. Behav. Biomed. Mater. 2020, 112, 104032. [Google Scholar] [CrossRef]
- Anguiano-Sanchez, J.; Martinez-Romero, O.; Siller, H.R.; Diaz-Elizondo, J.A.; Flores-Villalba, E.; Rodriguez, C.A. Influence of PEEK Coating on Hip Implant Stress Shielding: A Finite Element Analysis. Comput. Math. Methods Med. 2016, 2016, 6183679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoidis, P. The all-on-4 modified polyetheretherketone treatment approach: A clinical report. J. Prosthet. Dent. 2018, 119, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Parmigiani-Izquierdo, J.M.; Cabaña-Muñoz, M.E.; Merino, J.J.; Sánchez-Pérez, A. Zirconia implants and peek restorations for the replacement of upper molars. Int. J. Implant. Dent. 2017, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabello-Domínguez, G.; Pérez-López, J.; Veiga-López, B.; González, D.; Revilla-León, M. Maxillary zirconia and mandibular composite resin-lithium disilicate-modified PEEK fixed implant-supported restorations for a completely edentulous patient with an atrophic maxilla and mandible: A clinical report. J. Prosthet. Dent. 2020, 124, 403–410. [Google Scholar] [CrossRef]
- Qin, L.; Yao, S.; Zhao, J.; Zhou, C.; Oates, T.W.; Weir, M.D.; Wu, J.; Xu, H.H.K. Review on development and dental applications of polyetheretherketone-based biomaterials and restorations. Materials 2021, 14, 408. [Google Scholar] [CrossRef]
- Daubert, D.M.; Weinstein, B.F. Biofilm as a risk factor in implant treatment. Periodontol. 2000 2019, 81, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Mellado-Valero, A.; Buitrago-Vera, P.; Solá-Ruiz, M.F.; Ferrer-García, J.C. Decontamination of dental implant surface in peri-implantitis treatment: A literature review. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, e869–e876. [Google Scholar] [CrossRef] [PubMed]
- National Health Service (NHS.UK): “Antibiotics”. Available online: https://www.nhs.uk/conditions/antibiotics/side-effects/ (accessed on 23 May 2020).
- Allison, R.R.; Moghissi, K. Photodynamic therapy (PDT): PDT mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Konopka, K.; Goslinski, T. Photodynamic therapy in dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B 2009, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sibata, C.H.; Colussi, V.C.; Oleinick, N.L.; Kinsella, T.J. Photodynamic therapy: A new concept in medical treatment. Brazilian. J. Med. Biol. Res. 2000, 33, 869–880. [Google Scholar] [CrossRef] [Green Version]
- Gursoy, H.; Ozcakir-Tomruk, C.; Tanalp, J.; Yılmaz, S. Photodynamic therapy in dentistry: A literature review. Clin. Oral Investig. 2013, 17, 1113–1125. [Google Scholar] [CrossRef]
- Chiu, K.C.; Shih, Y.H.; Wang, T.H.; Lan, W.C.; Li, P.J.; Jhuang, H.S.; Hsia, S.M.; Shen, Y.W.; Chen, Y.-C.M.; Shieh, T.M. In vitro antimicrobial and antipro-inflammation potential of honokiol and magnolol against oral pathogens and macrophages. J. Formos. Med. Assoc. 2020. [Google Scholar] [CrossRef]
- Kyomoto, M.; Moro, T.; Yamane, S.; Hashimoto, M.; Takatori, Y.; Ishihara, K. Poly(ether-ether-ketone) orthopedic bearing surface modified by self-initiated surface grafting of poly(2-methacryloyloxyethyl phosphorylcholine). Biomaterials 2013, 34, 7829–7839. [Google Scholar] [CrossRef]
- Ma, R.; Tang, T. Current strategies to improve the bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef] [Green Version]
- Kurtz, S. PEEK Biomaterials Handbook, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Ma, X.; Li, F.; Cao, J.; Li, J.; Chen, H.; Zhao, C. Vickers microhardness and microstructure relationship of Ti-6Al-4V alloy under cyclic forward-reverse torsion and monotonic torsion loading. Mater. Des. 2017, 114, 271–281. [Google Scholar] [CrossRef]
- Wang, A.; Jones, I.P.; Landini, G.; Mei, J.; Tse, Y.Y.; Li, Y.X.; Ke, L.; Huang, Y.; Liu, L.I.; Wang, C.; et al. Backscattered electron imaging and electron backscattered diffraction in the study of bacterial attachment to titanium alloy structure. J. Microsc. 2018, 270, 53–63. [Google Scholar] [CrossRef]
- Barkarmo, S.; Longhorn, D.; Leer, K.; Johansson, C.B.; Stenport, V.; Franco-Tabares, S.; Kuehne, S.A.; Sammons, R. Biofilm formation on polyetheretherketone and titanium surfaces. Clin. Exp. Dent. Res. 2019, 5, 427–437. [Google Scholar] [CrossRef]
- De-la-Pinta, I.; Cobos, M.; Ibarretxe, J.; Montoya, E.; Eraso, E.; Guraya, T.; Quindós, G. Effect of biomaterials hydrophobicity and roughness on biofilm development. J. Mater. Sci. Mater. Med. 2019, 30, 77. [Google Scholar] [CrossRef]
- Meza-Siccha, A.S.; Aguilar-Luis, M.A.; Silva-Caso, W.; Mazulis, F.; Barragan-Salazar, C.; Del Valle-Mendoza, J. In Vitro Evaluation of Bacterial Adhesion and Bacterial Viability of Streptococcus mutans, Streptococcus sanguinis, and Porphyromonas gingivalis on the Abutment Surface of Titanium and Zirconium Dental Implants. Int. J. Dent. 2019, 2019, 4292976. [Google Scholar] [CrossRef] [Green Version]
- Han, A.; Tsoi, J.K.H.; Rodrigues, F.P.; Leprince, J.G.; Palin, W.M. Bacterial adhesion mechanisms on dental implant surfaces and the influencing factors. Int. J. Adhes. Adhes. 2016, 69, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Sperandio, F.F.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent. Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef] [Green Version]
- D’Ercole, S.; Cellini, L.; Pilato, S.; Di Lodovico, S.; Iezzi, G.; Piattelli, A.; Petrini, M. Material characterization and Streptococcus oralis adhesion on Polyetheretherketone (PEEK) and titanium surfaces used in implantology. J. Mater. Sci. Mater. Med. 2020, 31, 84. [Google Scholar] [CrossRef]
- Hahnel, S.; Wieser, A.; Lang, R.; Rosentritt, M. Biofilm formation on the surface of modern implant abutment materials. Clin. Oral Implant. Res. 2015, 26, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.-Y.; Shimoe, S.; Fuh, L.-J.; Lin, C.-K.; Lin, D.-J.; Kaku, M. Bonding and Thermal Cycling Performances of Two (Poly)Aryl–Ether–Ketone (PAEKs) Materials to an Acrylic Denture Base Resin. Polymers 2021, 13, 543. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Cheng, Q.; Zhao, R.; Zhu, X.; Yang, X.; Yang, X.; Zhang, K.; Song, Y.; Zhang, X. Comparison of osteointegration property between PEKK and PEEK: Effects of surface structure and chemistry. Biomaterials 2018, 170, 116–126. [Google Scholar] [CrossRef] [PubMed]







| Materials | Abbr. | Main Composition 1 | Manufacturer |
|---|---|---|---|
| Alloy material | |||
| ASTM F136 | Ti-6Al-4V | Ti, Al, V, others | Green Dentech Co. Ltd., Tainan, Taiwan |
| Ceramic material | |||
| 90X10-HT | Y-TZP 2 | ZrO2, Y2O3, others | Aidite Technology Co., Ltd., Qin Huang Dao, Mainland China |
| Polymer material | |||
| VESTAKEEP | PEEK 3 | Poly-ether-ether-ketone | Evonik Japan Co., Tokyo, Japan |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, T.-Y.; Lin, D.-J.; Mine, Y.; Tasi, C.-Y.; Li, P.-J.; Shih, Y.-H.; Chiu, K.-C.; Wang, T.-H.; Hsia, S.-M.; Shieh, T.-M. Biofilm Formation on the Surface of (Poly)Ether-Ether-Ketone and In Vitro Antimicrobial Efficacy of Photodynamic Therapy on Peri-Implant Mucositis. Polymers 2021, 13, 940. https://doi.org/10.3390/polym13060940
Peng T-Y, Lin D-J, Mine Y, Tasi C-Y, Li P-J, Shih Y-H, Chiu K-C, Wang T-H, Hsia S-M, Shieh T-M. Biofilm Formation on the Surface of (Poly)Ether-Ether-Ketone and In Vitro Antimicrobial Efficacy of Photodynamic Therapy on Peri-Implant Mucositis. Polymers. 2021; 13(6):940. https://doi.org/10.3390/polym13060940
Chicago/Turabian StylePeng, Tzu-Yu, Dan-Jae Lin, Yuichi Mine, Chi-Yang Tasi, Po-Jung Li, Yin-Hwa Shih, Kuo-Chou Chiu, Tong-Hong Wang, Shih-Min Hsia, and Tzong-Ming Shieh. 2021. "Biofilm Formation on the Surface of (Poly)Ether-Ether-Ketone and In Vitro Antimicrobial Efficacy of Photodynamic Therapy on Peri-Implant Mucositis" Polymers 13, no. 6: 940. https://doi.org/10.3390/polym13060940
APA StylePeng, T. -Y., Lin, D. -J., Mine, Y., Tasi, C. -Y., Li, P. -J., Shih, Y. -H., Chiu, K. -C., Wang, T. -H., Hsia, S. -M., & Shieh, T. -M. (2021). Biofilm Formation on the Surface of (Poly)Ether-Ether-Ketone and In Vitro Antimicrobial Efficacy of Photodynamic Therapy on Peri-Implant Mucositis. Polymers, 13(6), 940. https://doi.org/10.3390/polym13060940