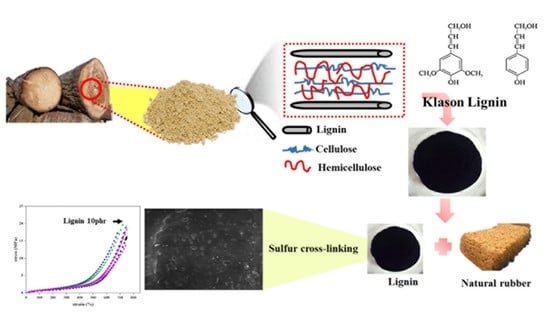
The Characteristics of Natural Rubber Composites with Klason Lignin as a Green Reinforcing Filler: Thermal Stability, Mechanical and Dynamical Properties
Abstract
:1. Introduction
2. Experimental
2.1. Material
2.2. Klason Lignin Extraction Method and Properties
2.3. Homogenization Process and Particle Size Distribution Analysis
2.4. Preparation of NR/Lignin Composites
2.5. Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.6. Mooney Viscosity and Cure Characterization and Bound Rubber Content of the Samples
2.7. Filler–Filler Interactions, Mechanical Properties and Thermal Aging Properties of the Samples
2.8. Morphological Characterization, Thermal and Dynamic Properties of the Samples
3. Results and Discussion
3.1. Properties of Extracted Klason Lignin
3.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.3. Mooney Viscosity
3.4. Curing Characteristics of the Samples
3.5. Dependence of Shear Modulus (G’) on Strain
3.6. Morphological Properties of the Samples
3.7. Mechanical Properties of the Samples
3.8. Thermogravimetric Analysis (TGA)
3.9. Dynamic Mechanical Analysis (DMA) of the Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bijarimi, M.; Zulkafli, H.; Beg, M. Mechanical properties of industrial tyre rubber compounds. J. Appl. Sci. 2010, 10, 1345–1348. [Google Scholar] [CrossRef] [Green Version]
- Phinyocheep, P. Chapter 3—Chemical modification of natural rubber (NR) for improved performance. In Chemistry, Manufacture and Applications of Natural Rubber; Kohjiya, S., Ikeda, Y., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 68–118. [Google Scholar]
- Ľudmila, H.; Michal, J.; Andrea, Š.; Aleš, H. Lignin, potential products and their market value. Wood Res. 2015, 60, 973–986. [Google Scholar]
- Gosselink, R.J.A.; Snijder, M.H.B.; Kranenbarg, A.; Keijsers, E.R.P.; de Jong, E.; Stigsson, L.L. Analytical protocols for characterisation of sulphur-free lignin. J. Appl. Polym. Sci. 2004, 20, 191–203. [Google Scholar] [CrossRef]
- Tran, C.D.; Chen, J.; Keum, J.K.; Naskar, A.K. A New Class of Renewable Thermoplastics with Extraordinary Performance from Nanostructured Lignin-Elastomers. Adv. Funct. Mater. 2016, 26, 2677–2685. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, F.P.; Marita, M.J.; Hatfield, D.R.; Ralph, A.S.; Christensen, H.J.; et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenylpropanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, F.J. Hydrogen bonding in lignin: A Fourier transform infrared model compound study. Biomacromolecules 2005, 6, 2815–2821. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, K.; Liu, Q.; Gu, Y.; Luo, Z.; Cen, K.; Fransson, T. Comparison of the pyrolysis behavior of lignins from different tree species. Biotechnol. Adv. 2009, 27, 562–567. [Google Scholar] [CrossRef]
- Minu, K.; Kurian Jiby, K.; Kishore, V.V.N. Isolation and purification of lignin and silica from the black liquor generated during the production of bioethanol from rice straw. Biomass Bioenergy 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Tejado, A.; Peňa, C.; Labidi, J.; Echevérria, J.M.; Mondragon, I. Physico chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef]
- Xia, Z.; Li, J.; Zhang, J.; Zhang, X.; Zheng, X.; Zhang, J. Processing and Valorization of Cellulose, Lignin and Lignocellulose Using Ionic Liquids. J. Bioresour. Bioprod. 2020, 5, 79–95. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Abdullah, D.K. Isolation and Characterization of Lignin from Rubber Wood in Ionic Liquid Medium. Mod. Appl. Sci. 2010, 4, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Ganesh Saratale, R.; Cho, S.-K.; Dattatraya Saratale, G.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Naresh Bharagava, R.; Kumar, G.; Su Kim, D.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Fukushima, K.; Kakehi, A. Formation and chemical structures of acid-soluble lignin h sulfuric acid treatment time and acid-soluble lignin content of hardwood. Wood Sci. 2001, 47, 69–72. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in green polymer composites from lignin for multifunctional applications: A review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Barana, D.; Ali, S.D.; Salanti, A.; Orlandi, M.; Castellani, L.; Hanel, T. Influence of lignin features on thermal stability and mechanical properties of natural rubber compounds. ACS Sustain. Chem. Eng. 2016, 4, 5258–5267. [Google Scholar] [CrossRef]
- Yu, P.; He, H.; Jia, Y.; Tian, S.; Chen, J.; Jia, D.; Luo, Y. A comprehensive study on lignin as a green alternative of silica in natural rubber composites. Polym. Test. 2016, 54, 176–185. [Google Scholar] [CrossRef]
- Bahl, K.; Miyoshi, T.; Jana, C. Hybrid fillers of lignin and carbon black for lowering of viscoelastic loss in rubber compounds. Polymer 2014, 55, 3825–3835. [Google Scholar] [CrossRef]
- Cao, X.V.; Ismail, H.; Rashid, A.A.; Takeichi, T.; Vo-Huu, T. Maleated natural rubber as a coupling agent for recycled high density polyethylene/natural rubber/kenaf powder biocomposites. Polym. Plast. Technol. Eng. 2012, 51, 904–910. [Google Scholar] [CrossRef]
- Xiao, S.; Feng, J.; Zhu, J.; Wang, X.; Yi, C.; Su, S. Preparation and characterization of lignin-layered double hydroxide/styrene-butadiene rubber composites. J. Appl. Polym. Sci. 2013, 130, 1308–1312. [Google Scholar] [CrossRef]
- Jiang, C.; He, H.; Yu, P.; Wang, D.K.; Zhou, L.; Jia, D.M. Plane-interface-induced lignin based nanosheets and its reinforcing effect on styrene-butadiene rubber. Express Polym. Lett. 2014, 8, 619–634. [Google Scholar] [CrossRef] [Green Version]
- Datta, J.; Parcheta, P.; Surówka, J. Softwood-lignin/natural rubber composites containing novel plasticizing agent: Preparation and plasticizing agent: Preparation and characterization. Ind. Crop. Prod. 2016, 95, 675–685. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; Huang, J.; Yang, D.; Qiu, X. Bioinspired engineering towards tailoring advanced lignin/rubber elastomers. Polymers 2018, 10, 1033. [Google Scholar] [CrossRef] [Green Version]
- Lang, J.M.; Shrestha, U.M.; Dadmun, M. The effect of plant source on the properties of lignin-based polyurethanes. Front. Energy Res. 2018, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Lia, H.; Lianga, Y.; Lia, P.; Heb, C. Conversion of biomass lignin to high-value polyurethane: A review. J. Bioresour. Bioprod. 2020, 5, 163–179. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. Controlling lignin particle size for polymer blend applications. J. Appl. Polym. Sci. 2017, 134, 44669. [Google Scholar] [CrossRef]
- Li, B.; You, S.; Qi, W.; Wang, Y.; Su, R.; He, Z. Structure-tunable assembly of lignin sub-micro spheres by modifying the amphiphilic interfaces of lignin via n-alkane. Europ. Polym. J. 2020, 106, 109539. [Google Scholar] [CrossRef]
- Mohamad Aini, N.A.; Othman, N.; Hussin, M.H.; Sahakora, K.; Hayeemasae, N. Hydroxymethylation-modified lignin and its effectiveness as a filler in rubber composites. Processes 2019, 7, 315. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; He, H.; Yao, X.; Yu, P.; Zhou, L.; Jia, D. The aggregation structure regulation of lignin by chemical modification and its effect on the property of lignin/styrene–butadiene rubber composites. J. Appl. Polym. Sci. 2018, 135, 45759. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, R.; Shen, D.; Zhang, H. Structural analysis of lignin residue from black liquor and its thermal performance in thermogravimetric-Fourier transform infrared spectroscopy. Bioresour. Technol. 2013, 128, 633–639. [Google Scholar] [CrossRef]
- Pandey, K. A study of chemical structure of soft and hard wood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Jiang, C.; He, H.; Yao, X.; Yu, P.; Zhou, L.; Jia, D. In situ dispersion and compatibilization of lignin/epoxidized natural rubber composites: Reactivity, morphology and property. J. Appl. Polym. Sci. 2015, 132, 1–10. [Google Scholar] [CrossRef]
- Hosseinmardi, A.; Amiralian, N.; Hayati, A.N.; Darren, J.M.; Pratheep, K.A. Toughening of natural rubber nanocomposites by the incorporation of nanoscale lignin combined with an industrially relevant leaching process. Ind. Crop. Prod. 2021, 159, 113063. [Google Scholar] [CrossRef]
- Xiong, W.; Yang, D.; Zhong, R.; Li, Y.; Zhou, H. Preparation of lignin-based silica composite submicron particles from alkali lignin and sodium silicate in aqueous solution using a direct precipitation method. Ind. Crop. Prod. 2015, 75, 285–292. [Google Scholar] [CrossRef]
- Ikeda, Y.; Phakkeeree, T.; Junkong, P.; Yokohama, H.; Phinyocheep, P.; Kitano, R.; Kato, A. Reinforcing biofiller “lignin” for high performance green natural rubber nanocomposites. RSC Adv. 2017, 7, 5222–5231. [Google Scholar] [CrossRef] [Green Version]
- Gregorová, A.; Košíková, B.; Moravčík, R. Stabilization effect of lignin in natural rubber. Polym. Degrad. Stab. 2006, 91, 229–233. [Google Scholar] [CrossRef]
- Košíková, B.; Gregorová, A.; Osvald, A.; Krajčovičová, J. Role of lignin filler in stabilization of natural rubber–based composites. J. Appl. Polym. Sci. 2007, 103, 1226–1231. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A. Materials produced from plant biomass. part III: Degradation kinetics and hydrogen bonding in lignin. Mater. Res. 2013, 16, 1065–1070. [Google Scholar] [CrossRef] [Green Version]
- Jacob, M.; Francis, B.; Varughese, K.T.; Thomas, S. Dynamical mechanical analysis of sisal/oil palm hybrid fiber-reinforced natural rubber composites. Polym. Compos. 2006, 27, 671–680. [Google Scholar]
- Sarkawi, S.S.; Aziz, A.K.C.; Rahim, R.A.; Ghani, R.A.; Kamaruddin, A.N. Properties of Epoxidized Natural Rubber Tread Compound: The Hybrid Reinforcing Effect of Silica and Silane System. Polym. Polym. Compos. 2016, 24, 775–782. [Google Scholar] [CrossRef]













| Sample Code | NR/BHT/Lignin |
|---|---|
| Unfilled | 100/0/0 |
| Control (BHT) | 100/1.5/0 |
| Lignin 1.5 | 100/0/1.5 |
| Lignin 5 | 100/0/5 |
| Lignin 10 | 100/0/10 |
| Lignin 15 | 100/0/15 |
| Lignin 20 | 100/0/20 |
| Band Position (cm−1) | Assignment |
|---|---|
| 3396–3406 | O–H Stretching |
| 2923 | C–H Stretching |
| 2849 | C–H Stretching |
| 1704 | C=O Stretching Unconjugated |
| 1614 | C=O Stretching Conjugated |
| 1461 | CH2 Deformation stretching |
| 1374 | C–O stretching of the syringyl ring |
| 1192 | C–C and C–O stretching vibration of guaiacyl ring |
| 1035 | C–H stretching of Aromatic in guaiacyl ring and C–O from primary alcohol |
| 1112 | C–H stretching in Aromatic deformation in the syringyl ring |
| 778 and 619 | C–H out of plane in positions 2, 5 and 6 (G units) |
| Lignin Content | Mooney Viscosity (ML 1 + 4 (100 °C)) |
|---|---|
| Control (BHT) | 37.5 |
| Lignin 0 phr | 40.9 |
| Lignin 1.5 phr | 39.1 |
| Lignin 5 phr | 38.7 |
| Lignin 10 phr | 35.2 |
| Lignin 15 phr | 35.1 |
| Lignin 20 phr | 34.9 |
| Formula | ts2 (min) | tc90 (min) | ML (dN m) | MH (dN m) | ΔM (dN m) |
|---|---|---|---|---|---|
| Control (BHT) | 0.49 | 1.41 | 2.36 | 12.31 | 9.95 |
| NR 0lignin | 0.45 | 1.41 | 2.17 | 11.78 | 9.61 |
| NR 1.5lignin | 0.51 | 1.51 | 2.36 | 12.84 | 10.48 |
| NR 5lignin | 0.55 | 1.57 | 2.33 | 13.31 | 10.98 |
| NR 10lignin | 1.02 | 2.13 | 2.44 | 14.29 | 11.85 |
| NR 15lignin | 1.10 | 2.37 | 2.53 | 14.55 | 12.02 |
| NR 20lignin | 0.52 | 2.06 | 2.62 | 15.27 | 12.65 |
| Lignin Content | Decomposition Temperature (T50) (°C) |
|---|---|
| Control (1.5 BHT) | 377.67 |
| Lignin 0 phr | 357.90 |
| Lignin 1.5 phr | 383.00 |
| Lignin 5 phr | 384.83 |
| Lignin 10 phr | 390.83 |
| Lignin 15 phr | 395.83 |
| Lignin 20 phr | 394.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intapun, J.; Rungruang, T.; Suchat, S.; Cherdchim, B.; Hiziroglu, S. The Characteristics of Natural Rubber Composites with Klason Lignin as a Green Reinforcing Filler: Thermal Stability, Mechanical and Dynamical Properties. Polymers 2021, 13, 1109. https://doi.org/10.3390/polym13071109
Intapun J, Rungruang T, Suchat S, Cherdchim B, Hiziroglu S. The Characteristics of Natural Rubber Composites with Klason Lignin as a Green Reinforcing Filler: Thermal Stability, Mechanical and Dynamical Properties. Polymers. 2021; 13(7):1109. https://doi.org/10.3390/polym13071109
Chicago/Turabian StyleIntapun, Jutharat, Thipsuda Rungruang, Sunisa Suchat, Banyat Cherdchim, and Salim Hiziroglu. 2021. "The Characteristics of Natural Rubber Composites with Klason Lignin as a Green Reinforcing Filler: Thermal Stability, Mechanical and Dynamical Properties" Polymers 13, no. 7: 1109. https://doi.org/10.3390/polym13071109
APA StyleIntapun, J., Rungruang, T., Suchat, S., Cherdchim, B., & Hiziroglu, S. (2021). The Characteristics of Natural Rubber Composites with Klason Lignin as a Green Reinforcing Filler: Thermal Stability, Mechanical and Dynamical Properties. Polymers, 13(7), 1109. https://doi.org/10.3390/polym13071109