Plasma-Assisted Synthesis of Multicomponent Nanoparticles Containing Carbon, Tungsten Carbide and Silver as Multifunctional Filler for Polylactic Acid Composite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
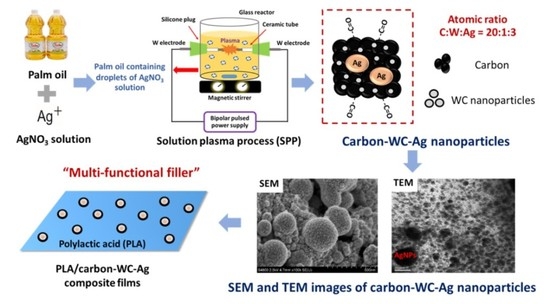
2.2. Preparation of Carbon-WC, Carbon-Ag and Carbon-WC-Ag Nanoparticles via SPP
2.3. Preparation of Neat PLA and PLA Composite Films
2.4. Characterization
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Carbon-WC and Carbon-WC-Ag Nanoparticles
3.2. Morphology of Neat PLA and PLA Composite Films
3.3. Thermogravimetric Analysis
3.4. Differential Scanning Calorimetry
3.5. X-ray Diffraction
3.6. Mechanical Test
3.7. Antibacterial Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fazita, M.R.N.; Jayaraman, K.; Bhattacharyya, D.; Hossain, M.S.; Haafiz, M.K.M.; Khalil, A. Disposal options of bamboo fabric-reinforced poly(Lactic) acid composites for sustainable packaging: Biodegradability and recyclability. Polymers 2015, 7, 1476–1496. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; Chen, Y.; Luo, P.; Chen, T. properties of luffa fiber reinforced PHBV biodegradable composites. Polymers 2019, 11, 1765. [Google Scholar] [CrossRef] [PubMed]
- Huh, M.; Jung, M.; Park, Y.; Kim, B.-J.; Kang, M.; Holden, P.; Yun, S. Effect of carbon nanotube functionalization on the structure and properties of poly(3-hydroxybutyrate)/MWCNTs biocomposites. Macromol. Res. 2014, 22, 765. [Google Scholar] [CrossRef]
- Li, T.; Sun, H.; Wu, B.; Han, H.; Li, D.; Wang, J.-K.; Zhang, J.; Huang, J.; Sun, D. High-performance polylactic acid composites reinforced by artificially cultured diatom frustules. Mater. Des. 2020, 195, 109003. [Google Scholar] [CrossRef]
- Le Phuong, H.A.; Izzati Ayob, N.A.; Blanford, C.F.; Mohammad Rawi, N.F.; Szekely, G. Nonwoven membrane supports from renewable resources: Bamboo fiber reinforced poly(Lactic Acid) composites. ACS Sustain. Chem. Eng. 2019, 7, 11885–11893. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Gao, J.; Wang, J. Facile fabrication of a homogeneous cellulose/polylactic acid composite film with improved biocompatibility, biodegradability and mechanical properties. Green Chem. 2019, 21, 4449–4456. [Google Scholar] [CrossRef]
- Gorrasi, G.; Milone, C.; Piperopoulos, E.; Lanza, M.; Sorrentino, A. Hybrid clay mineral-carbon nanotube-PLA nanocomposite films. Preparation and photodegradation effect on their mechanical, thermal and electrical properties. Appl. Clay Sci. 2013, 71, 49–54. [Google Scholar] [CrossRef]
- Khan, B.A.; Chevali, V.S.; Na, H.; Zhu, J.; Warner, P.; Wang, H. Processing and properties of antibacterial silver nanoparticle-loaded hemp hurd/poly(lactic acid) biocomposites. Compos. B Eng. 2016, 100, 10–18. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, H.; Liu, W.; Zhang, M.; Du, Z.; Wang, X. The synergistic effect of zinc oxide and phenylphosphonic acid zinc salt on the crystallization behavior of poly (lactic acid). Polym. Degrad. Stab. 2015, 122, 25–35. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Ellis, G. Non-isothermal cold-crystallization behavior and kinetics of poly(l-lactic Acid)/WS2 inorganic nanotube nanocomposites. Polymers 2015, 7, 2175–2189. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Ma, X.; Fang, J. Influence of carbon black on the properties of plasticized poly(lactic acid) composites. Polym. Degrad. Stab. 2008, 93, 1044–1052. [Google Scholar] [CrossRef]
- Zhou, Y.; Lei, L.; Yang, B.; Li, J.; Ren, J. Preparation and characterization of polylactic acid (PLA) carbon nanotube nanocomposites. Polym. Test 2018, 68, 34–38. [Google Scholar] [CrossRef]
- Pan, W.; Xiao, X.; Li, J.; Deng, S.; Shan, Q.; Yue, Y.; Tian, Y.; Nabar, N.R.; Wang, M.; Hao, L. The comparison of biocompatibility and osteoinductivity between multi-walled and single-walled carbon nanotube/PHBV composites. J. Mater. Sci. Mater. Med. 2018, 29, 189. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Liu, X.; Li, L. Biomass derived interconnected hierarchical micro-meso-macro- porous carbon with ultrahigh capacitance for supercapacitors. Carbon 2019, 147, 540–549. [Google Scholar] [CrossRef]
- Pontiroli, D.; Scaravonati, S.; Magnani, G.; Fornasini, L.; Bersani, D.; Bertoni, G.; Milanese, C.; Girella, A.; Ridi, F.; Verucchi, R.; et al. Super-activated biochar from poultry litter for high-performance supercapacitors. Microporous Mesoporous Mater. 2019, 285, 161–169. [Google Scholar] [CrossRef]
- Aup-Ngoen, K.; Noipitak, M. Effect of carbon-rich biochar on mechanical properties of PLA-biochar composites. Sustain. Chem. Pharm. 2020, 15, 100204. [Google Scholar] [CrossRef]
- Takagi, H.; Kako, S.; Kusano, K.; Ousaka, A. Thermal conductivity of PLA-bamboo fiber composites. Adv. Compos. Mater. 2007, 16, 377–384. [Google Scholar] [CrossRef]
- Yu, H.; Sun, B.; Zhang, D.; Chen, G.; Yang, X.; Yao, J. Reinforcement of biodegradable poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with cellulose nanocrystal/silver nanohybrids as bifunctional nanofillers. J. Mater. Chem. B 2014, 2, 8479–8489. [Google Scholar] [CrossRef]
- Bai, T.; Zhu, B.; Liu, H.; Wang, Y.; Song, G.; Liu, C.; Shen, C. Biodegradable poly(lactic acid) nanocomposites reinforced and toughened by carbon nanotubes/clay hybrids. Int. J. Biol. Macromol. 2020, 151, 628–634. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Iannoni, A.; Saino, E.; Visai, L.; Berglund, L.A.; Kenny, J.M. Multifunctional bionanocomposite films of poly(lactic acid), cellulose nanocrystals and silver nanoparticles. Carbohydr. Polym. 2012, 87, 1596–1605. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Yang, X.-Y.; Lu, F.-F.; Chen, G.-Y.; Yao, J.-M. Fabrication of multifunctional cellulose nanocrystals/poly(lactic acid) nanocomposites with silver nanoparticles by spraying method. Carbohydr. Polym. 2016, 140, 209–219. [Google Scholar] [CrossRef]
- Tsou, C.H.; Yao, W.H.; Lu, Y.C.; Tsou, C.Y.; Wu, C.S.; Chen, J.; Wang, R.Y.; Su, C.; Hung, W.S.; De Guzman, M.; et al. Antibacterial property and cytotoxicity of a poly(lactic acid)/nanosilver-doped multiwall carbon nanotube nanocomposite. Polymers 2017, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, H.-Y.; Wang, C.; Yao, J. Effect of silver contents in cellulose nanocrystal/silver nanohybrids on PHBV crystallization and property improvements. Carbohydr. Polym. 2017, 173, 7–16. [Google Scholar] [CrossRef]
- Kang, J.; Li, O.L.; Saito, N. Synthesis of structure-controlled carbon nano spheres by solution plasma process. Carbon 2013, 60, 292–298. [Google Scholar] [CrossRef]
- Matsuda, N.; Nakashima, T.; Kato, T.; Shiroishi, H. Synthesis of multiwall carbon nanotube-supported platinum catalysts by solution plasma processing for oxygen reduction in polymer electrolyte fuel cells. Electrochim. Acta 2014, 146, 73–78. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Nitrogen-doped carbon nanoparticles derived from acrylonitrile plasma for electrochemical oxygen reduction. Phys. Chem. Chem. Phys. 2015, 17, 6227–6232. [Google Scholar] [CrossRef]
- Nemoto, S.; Ueno, T.; Hieda, J.; Bratescu, M.A.; Saito, N.; Watthanaphanit, A. Simple introduction of carboxyl head group with alkyl spacer onto multiwalled carbon nanotubes by solution plasma process. Jpn. J. Appl. Phys. 2017, 56, 096202. [Google Scholar] [CrossRef]
- Saito, N.; Hieda, J.; Takai, O. Synthesis process of gold nanoparticles in solution plasma. Thin Solid Films 2009, 518, 912–917. [Google Scholar] [CrossRef]
- Tong, D.G.; Wu, P.; Su, P.K.; Wang, D.Q.; Tian, H.Y. Preparation of zinc oxide nanospheres by solution plasma process and their optical property, photocatalytic and antibacterial activities. Mater. Lett. 2012, 70, 94–97. [Google Scholar] [CrossRef]
- Kang, J.; Li, O.L.; Saito, N. A simple synthesis method for nano-metal catalyst supported on mesoporous carbon: The solution plasma process. Nanoscale 2013, 5, 6874–6882. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Chantaramethakul, J.; Chokradjaroen, C.; Ishizaki, T. In situ solution plasma synthesis of silver nanoparticles supported on nitrogen-doped carbons with enhanced oxygen reduction activity. Mater. Lett. 2019, 251, 135–139. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Fe–N-doped carbon-based composite as an efficient and durable electrocatalyst for the oxygen reduction reaction. RSC Adv. 2016, 6, 114553–114559. [Google Scholar] [CrossRef]
- Kim, D.-w.; Li, O.L.; Pootawang, P.; Saito, N. Solution plasma synthesis process of tungsten carbide on N-doped carbon nanocomposite with enhanced catalytic ORR activity and durability. RSC Adv. 2014, 4, 16813–16819. [Google Scholar] [CrossRef]
- Takai, O. Solution plasma processing (SPP). Pure Appl. Chem. 2008, 80, 2003–2011. [Google Scholar] [CrossRef]
- Prasertsung, I.; Damrongsakkul, S.; Saito, N. Degradation of β-chitosan by solution plasma process (SPP). Polym. Degrad. Stab. 2013, 98, 2089–2093. [Google Scholar] [CrossRef]
- Morishita, T.; Ueno, T.; Panomsuwan, G.; Hieda, J.; Yoshida, A.; Bratescu, M.A.; Saito, N. Fastest formation routes of nanocarbons in solution plasma processes. Sci. Rep. 2016, 6, 36880. [Google Scholar] [CrossRef]
- Salifairus, M.J.; Abd Hamid, S.B.; Soga, T.; Alrokayan, S.A.; Khan, H.A.; Rusop, M. Structural and optical properties of graphene from green carbon source via thermal chemical vapor deposition. J. Mater. Res. 2016, 31, 1947–1956. [Google Scholar] [CrossRef]
- Ishak, A.; Dayana, K.; Mamat, M.H.; Malek, M.F.; Rusop, M. Nano-structured amorphous carbon films using novel palm oil precursor for solar cell applications. Optik 2015, 126, 1610–1612. [Google Scholar] [CrossRef]
- Fonseca, C.; Ochoa, A.; Ulloa, M.T.; Alvarez, E.; Canales, D.; Zapata, P.A. Poly(lactic acid)/TiO2 nanocomposites as alternative biocidal and antifungal materials. Mater. Sci. Eng. C 2015, 57, 314–320. [Google Scholar] [CrossRef]
- Villani, M.; Consonni, R.; Canetti, M.; Bertoglio, F.; Iervese, S.; Bruni, G.; Visai, L.; Iannace, S.; Bertini, F. Polyurethane-Based Composites: Effects of Antibacterial Fillers on the Physical-Mechanical Behavior of Thermoplastic Polyurethanes. Polymers 2020, 12, 362. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.-H.; Williams, C.J.; Derby, B.; Szekely, G. Oil-in-water separation with graphene-based nanocomposite membranes for produced water treatment. J. Membr. Sci. 2020, 603, 118007. [Google Scholar] [CrossRef]
- Rubin, H.N.; Neufeld, B.H.; Reynolds, M.M. Surface-Anchored Metal–Organic Framework–Cotton Material for Tunable Antibacterial Copper Delivery. ACS Appl. Mater. Interfaces 2018, 10, 15189–15199. [Google Scholar] [CrossRef]
- Al Aani, S.; Gomez, V.; Wright, C.J.; Hilal, N. Fabrication of antibacterial mixed matrix nanocomposite membranes using hybrid nanostructure of silver coated multi-walled carbon nanotubes. Chem. Eng. J. 2017, 326, 721–736. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, X.; Qiu, X.; Chen, J. Synthesis and electrical properties of uniform silver nanoparticles for electronic applications. J. Mater. Sci. 2009, 44, 1076–1081. [Google Scholar] [CrossRef]
- Zakaria, M.A.; Menazea, A.A.; Mostafa, A.M.; Al-Ashkar, E.A. Ultra-thin silver nanoparticles film prepared via pulsed laser deposition: Synthesis, characterization, and its catalytic activity on reduction of 4-nitrophenol. Surf. Interfaces 2020, 19, 100438. [Google Scholar] [CrossRef]
- Boca, S.C.; Potara, M.; Gabudean, A.M.; Juhem, A.; Baldeck, P.L.; Astilean, S. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Lett. 2011, 311, 131–140. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Al-Deyab, S.S. Antimicrobial activity of silver/starch/polyacrylamide nanocomposite. Int. J. Biol. Macromol. 2014, 68, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Cañamares, M.V.; Garcia-Ramos, J.V.; Gómez-Varga, J.D.; Domingo, C.; Sanchez-Cortes, S. Comparative study of the morphology, aggregation, adherence to glass, and surface-enhanced Raman scattering activity of silver nanoparticles prepared by chemical reduction of Ag+ using citrate and hydroxylamine. Langmuir 2005, 21, 8546–8553. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, L.; Zhang, T.; Li, K. Facile synthesis of Ag nanoparticles supported on MWCNTs with favorable stability and their bactericidal properties. J. Hazard. Mater. 2011, 187, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Munstedt, H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 2005, 26, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Qiao, Y.; Wang, Y.; Yan, Y.; Huang, J. Self-assembled laminated nanoribbon-directed synthesis of noble metallic nanoparticle-decorated silica nanotubes and their catalytic applications. J. Mater. Chem. 2012, 22, 18314–18320. [Google Scholar] [CrossRef]
- Bhaduri, B.; Polubesova, T. Facile synthesis of carbon-supported silver nanoparticles as an efficient reduction catalyst for aqueous 2-methyl-p-nitrophenol. Mater. Lett. 2020, 267, 127546. [Google Scholar] [CrossRef]
- Vijayakumar, P.S.; Prasad, B.L. Intracellular biogenic silver nanoparticles for the generation of carbon supported antiviral and sustained bactericidal agents. Langmuir 2009, 25, 11741–11747. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, P.; Soni, H. Catalytic reduction of nitrophenols using silver nanoparticles-supported activated carbon derived from agro-waste. J. Environ. Chem. Eng. 2018, 6, 28–36. [Google Scholar] [CrossRef]
- Surudžić, R.; Janković, A.; Bibić, N.; Vukašinović-Sekulić, M.; Perić-Grujić, A.; Mišković-Stanković, V.; Park, S.J.; Rhee, K.Y. Physico–chemical and mechanical properties and antibacterial activity of silver/poly(vinyl alcohol)/graphene nanocomposites obtained by electrochemical method. Compos. B Eng. 2016, 85, 102–112. [Google Scholar] [CrossRef]
- Gan, L.; Geng, A.; Wu, Y.; Wang, L.; Fang, X.; Xu, L.; Mei, C. Antibacterial, flexible, and conductive membrane based on MWCNTs/Ag coated electro-Spun PLA nanofibrous scaffolds as wearable fabric for body motion sensing. Polymers 2020, 12, 120. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, H.; Fang, C.; Ai, L.; Chen, J.; Su, J.; Zhang, Q.; Fu, Q. Facile synthesis of reduced graphene oxide/silver nanoparticles composites and their application for detecting heavy metal ions. J. Alloy. Compd. 2019, 787, 683–693. [Google Scholar] [CrossRef]
- Tammeveski, L.; Erikson, H.; Sarapuu, A.; Kozlova, J.; Ritslaid, P.; Sammelselg, V.; Tammeveski, K. Electrocatalytic oxygen reduction on silver nanoparticle/multi-walled carbon nanotube modified glassy carbon electrodes in alkaline solution. Electrochem. Commun. 2012, 20, 15–18. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Castro-Aguirre, E.; Auras, R. Thermal properties of ZnO and bimetallic Ag–Cu alloy reinforced poly(lactic acid) nanocomposite films. J. Therm. Anal. Calorim. 2016, 125, 205–214. [Google Scholar] [CrossRef]
- Abdul Rahaman, M.H.; Khandaker, M.U.; Khan, Z.R.; Kufian, M.Z.; Noor, I.S.M.; Arof, A.K. Effect of gamma irradiation on poly(vinyledene difluoride)–lithium bis(oxalato)borate electrolyte. Phys. Chem. Chem. Phys. 2014, 16, 11527–11537. [Google Scholar] [CrossRef] [PubMed]
- Janpetch, N.; Saito, N.; Rujiravanit, R. Fabrication of bacterial cellulose-ZnO composite via solution plasma process for antibacterial applications. Carbohydr. Polym. 2016, 148, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Bataev, I.; Oda, H.; Hokamoto, K. Synthesis of metastable cubic tungsten carbides by electrical explosion of tungsten wire in liquid paraffin. Adv. Powder Technol. 2018, 29, 2447–2455. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, D.; Zhou, J.; Xie, Z.; Xia, Y. Synthesis and characterization of tungsten carbide and application to electrocatalytic hydrogen evolution. RSC Adv. 2016, 6, 76307–76311. [Google Scholar] [CrossRef]
- Kim, H.; Saito, N. One-pot synthesis of purple benzene-derived MnO2-carbon hybrids and synergistic enhancement for the removal of cationic dyes. Sci. Rep. 2018, 8, 4342. [Google Scholar] [CrossRef]
- Cam, D.; Marucci, M. Influence of residual monomers and metals on poly (l-lactide) thermal stability. Polymer 1997, 38, 1879–1884. [Google Scholar] [CrossRef]
- Fan, Y.; Nishida, H.; Mori, T.; Shirai, Y.; Endo, T. Thermal degradation of poly(l-lactide): Effect of alkali earth metal oxides for selective l,l-lactide formation. Polymer 2004, 45, 1197–1205. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.P.; Madras, G. Thermal degradation of water soluble polymers and their binary blends. J. Appl. Polym. Sci. 2006, 101, 233–240. [Google Scholar] [CrossRef]
- Doganay, D.; Coskun, S.; Kaynak, C.; Unalan, H.E. Electrical, mechanical and thermal properties of aligned silver nanowire/polylactide nanocomposite films. Compos. B Eng. 2016, 99, 288–296. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Dominici, F.; Jiménez, A.; del Carmen Garrigós, M.; Kenny, J.M. Influence of thymol and silver nanoparticles on the degradation of poly(lactic acid) based nanocomposites: Thermal and morphological properties. Polym. Degrad. Stab. 2014, 108, 158–165. [Google Scholar] [CrossRef]
- Piekarska, K.; Sowinski, P.; Piorkowska, E.; Haque, M.M.U.; Pracella, M. Structure and properties of hybrid PLA nanocomposites with inorganic nanofillers and cellulose fibers. Compos. Part A Appl. Sci. Manuf. 2016, 82, 34–41. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.-n.; Huang, Z.-g.; Weng, Y.-x. Heat resistance, crystallization behavior, and mechanical properties of polylactide/nucleating agent composites. Mater. Des. (1980–2015) 2015, 66, 7–15. [Google Scholar] [CrossRef]
- Dai, X.; Cao, Y.; Shi, X.; Wang, X. Non-isothermal crystallization kinetics, thermal degradation behavior and mechanical properties of poly(lactic acid)/MOF composites prepared by melt-blending methods. RSC Adv. 2016, 6, 71461–71471. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Wu, G.-H.; Xiao, Y.-C.; Guo, H.-X.; Shao, F.-J. Crystallization behavior and mechanical properties of poly(lactic acid) complex fiber toughened by carbon nanotube nanocapsules. Text. Res. J. 2017, 88, 1616–1627. [Google Scholar] [CrossRef]
- Mat Desa, M.S.Z.; Hassan, A.; Arsad, A.; Mohammad, N.N.B. Mechanical properties of poly(lactic acid)/multiwalled carbon nanotubes nanocomposites. Mater. Res. Innov. 2014, 18 (Suppl. 6), S6-14–S6-17. [Google Scholar] [CrossRef]
- Castle, A.B.; Gracia-Espino, E.; Nieto-Delgado, C.; Terrones, H.; Terrones, M.; Hussain, S. Hydroxyl-functionalized and N-Doped multiwalled carbon nanotubes decorated with silver nanoparticles preserve cellular function. ACS Nano 2011, 5, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Hwang, J.; Kim, J.; Jeong, Y.; Hwang, M.P.; Choi, J. Antibacterial activity and cytotoxicity of multi-walled carbon nanotubes decorated with silver nanoparticles. Int. J. Nanomed. 2014, 9, 4621–4629. [Google Scholar]






| Fillers (g) | PLA (g) | Chloroform (mL) | Filler Content in PLA Composite Films (wt%) |
|---|---|---|---|
| 0.01 | 4.00 | 100.00 | 0.25 |
| 0.03 | 4.00 | 100.00 | 0.75 |
| 0.05 | 4.00 | 100.00 | 1.23 |
| 0.07 | 4.00 | 100.00 | 1.72 |
| 0.09 | 4.00 | 100.00 | 2.20 |
| Type of Elements | Atomic Percentage (%) ± SD | |||
|---|---|---|---|---|
| Activated Charcoal | Carbon-WC | Carbon-Ag | Carbon-WC-Ag | |
| Carbon (C) | 94.42 ± 0.16 | 91.15 ± 0.46 | 82.10 ± 1.44 | 75.35 ± 2.04 |
| Oxygen (O) | 5.58 ± 0.16 | 5.35 ± 0.72 | 7.08 ± 0.85 | 9.64 ± 1.60 |
| Tungsten (W) | - | 3.59 ± 1.07 | - | 3.81 ± 0.83 |
| Silver (Ag) | - | - | 10.81 ± 1.06 | 11.20 ± 1.18 |
| Composite Films | Tint (°C) | T50 (°C) | Tmax (°C) |
|---|---|---|---|
| Neat PLA | 102.4 a | 360.7 a | 366.5 a |
| PLA/activated charcoal | 104.9 a | 358.9 a,b | 364.8 a,b |
| PLA/carbon-WC | 107.1 a | 355.8 a | 363.3 a |
| PLA/carbon-Ag | 111.0 a | 359.5 a,b | 365.7 a |
| PLA/carbon-WC-Ag | 112.1 a | 357.8 a | 364.6 a,b |
| Composite Films | Tg (°C) | Tcc (°C) | Tm (°C) |
|---|---|---|---|
| Neat PLA | 60.1 b | 124.8 a | 152.0 a |
| PLA/activated charcoal | 59.6 b | - | 150.7 b |
| PLA/carbon-WC | 59.9 b | 118.1 a | 150.6 b |
| PLA/carbon-Ag | 59.8 b | 123.9 a | 150.7 b |
| PLA/carbon-WC-Ag | 59.3 b | 117.1 a | 150.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonyeun, N.; Rujiravanit, R.; Saito, N. Plasma-Assisted Synthesis of Multicomponent Nanoparticles Containing Carbon, Tungsten Carbide and Silver as Multifunctional Filler for Polylactic Acid Composite Films. Polymers 2021, 13, 991. https://doi.org/10.3390/polym13070991
Boonyeun N, Rujiravanit R, Saito N. Plasma-Assisted Synthesis of Multicomponent Nanoparticles Containing Carbon, Tungsten Carbide and Silver as Multifunctional Filler for Polylactic Acid Composite Films. Polymers. 2021; 13(7):991. https://doi.org/10.3390/polym13070991
Chicago/Turabian StyleBoonyeun, Nichapat, Ratana Rujiravanit, and Nagahiro Saito. 2021. "Plasma-Assisted Synthesis of Multicomponent Nanoparticles Containing Carbon, Tungsten Carbide and Silver as Multifunctional Filler for Polylactic Acid Composite Films" Polymers 13, no. 7: 991. https://doi.org/10.3390/polym13070991
APA StyleBoonyeun, N., Rujiravanit, R., & Saito, N. (2021). Plasma-Assisted Synthesis of Multicomponent Nanoparticles Containing Carbon, Tungsten Carbide and Silver as Multifunctional Filler for Polylactic Acid Composite Films. Polymers, 13(7), 991. https://doi.org/10.3390/polym13070991