Application of Redox-Responsive Hydrogels Based on 2,2,6,6-Tetramethyl-1-Piperidinyloxy Methacrylate and Oligo(Ethyleneglycol) Methacrylate in Controlled Release and Catalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hydrogels
2.3. Electrochemical Measurements
2.4. Ultra-High-Performance Liquid Chromatography (UHPLC)–Electrospray Ionization (ESI) Mass Spectrometry Analysis
2.5. Gas Chromatography (GC)–Mass Spectrometry (MS) Analysis
2.6. Fourier-Transform Infrared (FTIR)
3. Results
3.1. Synthesis of Hydrogels
3.2. Encapsulation-Release of Aspirin from P(TEMPO+-r-OEGMA) Hydrogels
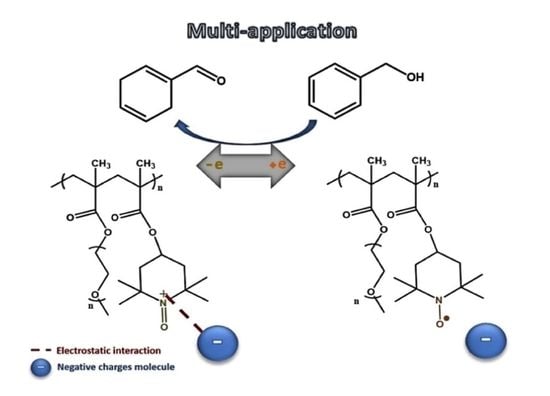
3.3. P(TEMPO-r-OEGMA) Hydrogels as Catalytic Scaffolds for the Oxidation of Alcohols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, N. Preparation methods and properties of hydrogel: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 112–117. [Google Scholar]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Chai, G.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wichterle, O.; Lim, D. Hydrophilic gels in biologic use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Echeverria, C.; Fernandes, S.; Godinho, M.; Borges, J.; Soares, P. Functional stimuli-responsive gels: Hydrogels and microgels. Gels 2018, 4, 54. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Urban, M.W. Recent advances and challenges in designing stimuli-responsive polymers. Prog. Polym. Sci. 2010, 35, 3–23. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Buwalda, S.J. Bio-based composite hydrogels for biomedical applications. Multifunct. Mater. 2020, 3, 022001. [Google Scholar] [CrossRef]
- Rohani Rad, E.; Vahabi, H.; Formela, K.; Saeb, M.R.; Thomas, S. Injectable poloxamer/graphene oxide hydrogels with well-controlled mechanical and rheological properties. Polym. Adv. Technol. 2019, 30, 2250–2260. [Google Scholar] [CrossRef]
- Rastin, H.; Zhang, B.; Mazinani, A.; Hassan, K.; Bi, J.; Tung, T.; Losic, D. 3D bioprinting of cell-laden electroconductive MXene nanocomposite bioinks. Nanoscale 2020, 12, 16069–16080. [Google Scholar] [CrossRef]
- Rezanejade, G.; Ghavami, S.; Sadat, S. A review on pH and temperature responsive gels in drug delivery. J. Chem Rev. 2020, 2, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Gupta, T.; Pradhan, A.; Bandyopadhyay-Ghosh, S.; Ghosh, S.B. Thermally exfoliated graphene oxide reinforced stress responsive conductive nanocomposite hydrogel. Polym. Adv. Technol. 2019, 30, 2392–2401. [Google Scholar] [CrossRef]
- Reddy, N.N.; Mohan, Y.M.; Varaprasad, K.; Ravindra, S.; Joy, P.A.; Raju, K.M. Magnetic and electric responsive hydrogel–magnetic nanocomposites for drug-delivery application. J. Appl. Polym. Sci. 2011, 122, 1364–1375. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, B.; Zhou, Q.; Jian, Y.; Le, X.; Lu, H. Ionic strength and thermal dual-responsive bilayer hollow spherical hydrogel actuator. Macromol. Rapid Commun. 2020, 41, 1900543. [Google Scholar] [CrossRef]
- Tao, N.; Zhang, D.; Li, X.; Lou, D.; Sun, X.; Wei, C.; Li, J.; Yang, J.; Liu, Y.N. Near-infrared light-responsive hydrogels via peroxide-decorated MXene-initiated polymerization. Chem. Sci. 2019, 10, 10765–10771. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.K.; Kasi, R.M.; Kim, S.C.; Sharma, N.; Zhou, Y. Stimuli-responsive polymer gels. Soft Matter 2008, 4, 1151–1157. [Google Scholar] [CrossRef]
- Sui, X.; Feng, X.; Hempenius, M.A.; Vancso, G.J. Redox active gels: Synthesis, structures and applications. J. Mater. Chem. B 2013, 1, 1658–1672. [Google Scholar] [CrossRef]
- Zoetebier, B.; Hempenius, M.A.; Vancso, G.J. Redox-responsive organometallic hydrogels for in situ metal nanoparticle synthesis. Chem. Commun. 2015, 51, 636–639. [Google Scholar] [CrossRef]
- Blinco, J.P.; Hodgson, J.L.; Morrow, B.J.; Walker, J.R.; Will, G.D.; Coote, M.L.; Bottle, S.E. Experimental and theoretical studies of the redox potentials of cyclic nitroxides. J. Org. Chem. 2008, 73, 6763–6771. [Google Scholar] [CrossRef]
- Hansen, K.A.; Blinco, J.P. Nitroxide radical polymers—A versatile material class for high-tech applications. Polym. Chem. 2018, 9, 1479–1516. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Kuramochi, K.; Binh Vong, L.; Nagasaki, Y. Development of nitroxide radicals–containing polymer for scavenging reactive oxygen species from cigarette smoke. Sci. Technol. Adv. Mater. 2014, 15, 035002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janoschka, T.; Morgenstern, S.; Hiller, H.; Friebe, C.; Wolkersdörfer, K.; Häupler, B.; Hager, M.D.; Schubert, U.S. Synthesis and characterization of TEMPO- and viologen-polymers for water-based redox-flow batteries. Polym. Chem. 2015, 6, 7801–7811. [Google Scholar] [CrossRef]
- Vlad, A.; Singh, N.; Rolland, J.; Melinte, S.; Ajayan, P.M.; Gohy, J.F. Hybrid supercapacitor-battery materials for fast electrochemical charge storage. Sci. Rep. 2014, 4, 4315. [Google Scholar] [CrossRef] [Green Version]
- Khodeir, M.; Ernould, B.; Brassinne, J.; Ghiassinejad, S.; Jia, H.; Antoun, S.; Friebe, C.; Schubert, U.S.; Kochovski, Z.; Lu, Y.; et al. Synthesis and characterisation of redox hydrogels based on stable nitroxide radicals. Soft Matter 2019, 15, 6418–6426. [Google Scholar] [CrossRef] [PubMed]
- Khodeir, M.; Antoun, S.; Van Ruymbeke, E.; Gohy, J.F. Temperature and redox-responsive hydrogels based on nitroxide radicals and oligoethyleneglycol methacrylate. Macromol. Chem. Phys. 2020, 221, 1900550. [Google Scholar] [CrossRef]
- Cross, E.R. The electrochemical fabrication of hydrogels: A short review. SN Appl. Sci. 2020, 2, 397. [Google Scholar] [CrossRef] [Green Version]
- Kleber, C.; Lienkamp, K.; Rühe, J.; Asplund, M. Electrochemically controlled drug release from a conducting polymer hydrogel (PDMAAp/PEDOT) for local therapy and bioelectronics. Adv. Healthc. Mater. 2019, 8, 1801488. [Google Scholar] [CrossRef]
- Liu, R.; Dong, C.; Liang, X.; Wang, X.; Hu, X. Highly efficient catalytic aerobic oxidations of benzylic alcohols in water. J. Org. Chem. 2005, 70, 729–731. [Google Scholar] [CrossRef]
- Bobbitt, J.M. Oxoammonium salts. 6. 4-Acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium perchlorate: A stable and convenient reagent for the oxidation of alcohols. Silica gel catalysis. J. Org. Chem. 1998, 63, 9367–9374. [Google Scholar] [CrossRef]
- Gharehkhani, S.; Zhang, Y.; Fatehi, P. Lignin-derived platform molecules through TEMPO catalytic oxidation strategies. Prog. Energy Combust. Sci. 2019, 72, 59–89. [Google Scholar] [CrossRef]
- Karimi, B.; Farhangi, E. A Highly recyclable magnetic core-shell nanoparticle-supported TEMPO catalyst for efficient metal- and halogen-free aerobic oxidation of alcohols in water. Chem. Eur. J. 2011, 17, 6056–6060. [Google Scholar] [CrossRef]
- Bocian, A.; Gorczyński, A.; Marcinkowski, D.; Witomska, S.; Kubicki, M.; Mech, P.; Bogunia, M.; Brzeski, J.; Makowski, M.; Pawluć, P.; et al. New benzothiazole based copper(II) hydrazone Schiff base complexes for selective and environmentally friendly oxidation of benzylic alcohols: The importance of the bimetallic species tuned by the choice of the counterion. J. Mol. Liq. 2020, 302, 112590. [Google Scholar] [CrossRef]
- Mahmoudi, B.; Rostami, A.; Kazemnejadi, M.; Hamah-Ameen, B.A. Catalytic oxidation of alcohols and alkyl benzenes to carbonyls using Fe3O4@SiO2@(TEMPO)-co-(Chlorophyll-CoIII) as a bi-functional, self-co-oxidant nanocatalyst. Green Chem. 2020, 22, 6600–6613. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Vyavahare, N.R.; Kulkarni, M.G.; Mashelkar, R.A. Zero order release from glassy hydrogels. J. Memb. Sci. 1990, 54, 205–220. [Google Scholar] [CrossRef]




| Entry | TEMPO [mol%] | Co-Catalyst | Condition | T (°C) | t (hour) | Conversion (%) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 0.2 | TBN * | O2 | 50 | 1.5 | 100 | This work |
| 2 | 0.2 | TBN | O2 | 50 | 4 | 100 | [31] |
| 3 | 0.3 | NaNO2, DBDMH * | Air (0.9 MPa) | 80 | 1.5 | 99.8 | [28] |
| 4 | 5 | Cu, K2CO3 | Air | 40 | 24 | 100 | [32] |
| 5 | 0.68 | Co | Air | RT | 0.5 | 98 | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodeir, M.; Jia, H.; Vlad, A.; Gohy, J.-F. Application of Redox-Responsive Hydrogels Based on 2,2,6,6-Tetramethyl-1-Piperidinyloxy Methacrylate and Oligo(Ethyleneglycol) Methacrylate in Controlled Release and Catalysis. Polymers 2021, 13, 1307. https://doi.org/10.3390/polym13081307
Khodeir M, Jia H, Vlad A, Gohy J-F. Application of Redox-Responsive Hydrogels Based on 2,2,6,6-Tetramethyl-1-Piperidinyloxy Methacrylate and Oligo(Ethyleneglycol) Methacrylate in Controlled Release and Catalysis. Polymers. 2021; 13(8):1307. https://doi.org/10.3390/polym13081307
Chicago/Turabian StyleKhodeir, Miriam, He Jia, Alexandru Vlad, and Jean-François Gohy. 2021. "Application of Redox-Responsive Hydrogels Based on 2,2,6,6-Tetramethyl-1-Piperidinyloxy Methacrylate and Oligo(Ethyleneglycol) Methacrylate in Controlled Release and Catalysis" Polymers 13, no. 8: 1307. https://doi.org/10.3390/polym13081307
APA StyleKhodeir, M., Jia, H., Vlad, A., & Gohy, J. -F. (2021). Application of Redox-Responsive Hydrogels Based on 2,2,6,6-Tetramethyl-1-Piperidinyloxy Methacrylate and Oligo(Ethyleneglycol) Methacrylate in Controlled Release and Catalysis. Polymers, 13(8), 1307. https://doi.org/10.3390/polym13081307