The Study of pH Effects on Phase Transition of Multi-Stimuli Responsive P(NiPAAm-co-AAc) Hydrogel Using 2D-COS
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Analysis of the Temperature-Dependent IR Spectra of P(NiPAAm-co-AAc) Hydrogels with Different pHs
3.2. Determination of Transition Temperature of P(NiPAAm-co-AAc) Hydrogel with Different pHs
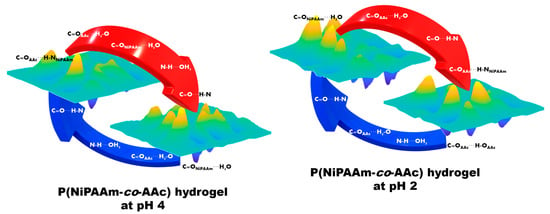
3.3. The Phase Transition Mechanism of P(NiPiPAAm-co-AAc) Hydrogels with Different pHs during the Heating and Cooling Processes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.; Xu, F. Bioactuators based on stimulus-responsive hydrogels and their emerging biomedical applications. NPG Asia Mater. 2019, 11, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Xing, Z.; Chen, M.; Yu, K.; Fu, Y.Q. Solvent-aided phase separation in hydrogel towards significantly enhanced mechanoresponsive strength. Acta Mech. Sin. 2021, 1–10. [Google Scholar] [CrossRef]
- Lu, H.; Xing, Z.; Hossain, M.; Leng, J. Scaling dynamics of globule-to-coil phase transition in double-network hydrogel with ultra-high stretchable strength. Smart Mater. Struct. 2020, 29, 085050. [Google Scholar] [CrossRef]
- Peponi, L.; Arrieta, M.P.; Mujica-Garcia, A.; López, D. 6-Smart Polymers. In Modification of Polymer Properties; Jasso-Gastinel, C.F., Kenny, J.M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 131–154. [Google Scholar]
- Aguilar, M.R.; San Román, J. Chapter 1—Introduction to Smart Polymers and Their Applications. In Smart Polymers and Their Applications, 2nd ed.; Aguilar, M.R., San Román, J., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 1–11. [Google Scholar]
- Dong, Y.; Wang, S.; Ke, Y.; Ding, L.; Zeng, X.; Magdassi, S.; Long, Y. 4D Printed Hydrogels: Fabrication, Materials, and Applications. Adv. Mater. Technol. 2020, 5, 2000034. [Google Scholar] [CrossRef]
- Frazar, E.M.; Shah, R.A.; Dziubla, T.D.; Hilt, J.Z. Multifunctional temperature-responsive polymers as advanced biomaterials and beyond. J. Appl. Polym. Sci. 2019, 137, 48770. [Google Scholar] [CrossRef] [Green Version]
- Schattling, P.; Jochum, F.D.; Theato, P. Multi-stimuli responsive polymers—The all-in-one talents. Polym. Chem. 2014, 5, 25–36. [Google Scholar] [CrossRef]
- Fu, X.; Hosta-Rigau, L.; Chandrawati, R.; Cui, J. Multi-Stimuli-Responsive Polymer Particles, Films, and Hydrogels for Drug Delivery. Chem 2018, 4, 2084–2107. [Google Scholar] [CrossRef] [Green Version]
- Hatai, J.; Hirschhäuser, C.; Niemeyer, J.; Schmuck, C. Multi-Stimuli-Responsive Supramolecular Polymers Based on Noncovalent and Dynamic Covalent Bonds. ACS Appl. Mater. Interfaces 2020, 12, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, J.; Guo, X.; Yang, S.; Ozen, M.O.; Chen, P.; Liu, X.; Du, W.; Xiao, F.; Demirci, U.; et al. Multi-stimuli-responsive programmable biomimetic actuator. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Shieh, Y.-T.; Lin, P.-Y.; Chen, T.; Kuo, S.-W. Temperature-, pH- and CO₂-Sensitive Poly (N-isopropylacryl amide-co-acrylic acid) Copolymers with High Glass Transition Temperatures. Polymers 2016, 8, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, S.; Kawano, R.; Onoe, H. Stimuli-responsive hydrogel microfibers with controlled anisotropic shrinkage and cross-sectional geometries. Soft Matter 2017, 13, 3710–3719. [Google Scholar] [CrossRef]
- Cao, Z.-Q.; Wang, G.-J. Multi-Stimuli-Responsive Polymer Materials: Particles, Films, and Bulk Gels. Chem. Rec. 2016, 16, 1398–1435. [Google Scholar] [CrossRef] [PubMed]
- Pham, S.H.; Choi, Y.; Choi, J. Stimuli-Responsive Nanomaterials for Application in Antitumor Therapy and Drug Delivery. Pharmaceutics 2020, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rad, E.R.; Vahabi, H.; Formela, K.; Saeb, M.R.; Thomas, S. Injectable poloxamer/graphene oxide hydrogels with well-controlled mechanical and rheological properties. Polym. Adv. Technol. 2019, 30, 2250–2260. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-L.; Kan, C.-W. Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Hu, J.; Yin, J.; Liu, S. Click Coupling Fullerene onto Thermoresponsive Water-Soluble Diblock Copolymer and Homopolymer Chains at Defined Positions. Macromolecules 2009, 42, 5007–5016. [Google Scholar] [CrossRef]
- Ahiabu, A.; Serpe, M.J. Rapidly Responding pH- and Temperature-Responsive Poly (N-Isopropylacrylamide)-Based Microgels and Assemblies. ACS Omega 2017, 2, 1769–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lue, S.J.; Chen, C.-H.; Shih, C.-M. Tuning of Lower Critical Solution Temperature (LCST) of Poly (N-Isopropylacrylamide-co-Acrylic acid) Hydrogels. J. Macromol. Sci. Part B 2011, 50, 563–579. [Google Scholar] [CrossRef]
- Sponchioni, M.; Palmiero, U.C.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, M.; Zahedi, P.; Abdouss, M.; Zarandi, M.A.; Manouchehri, S.; Mozdoori, N. Electrospun poly (N-isopropylacrylamide-co-acrylic acid)/cellulose laurate blend nanofibers containing adapalene: Morphology, drug release, and cell culture studies. Int. J. Polym. Mater. 2015, 65, 477–486. [Google Scholar] [CrossRef]
- Augustine, R.; Kim, D.-K.; Kalva, N.; Eom, K.H.; Kim, J.H.; Kim, I. Multi-stimuli-responsive nanomicelles fabricated using synthetic polymer polylysine conjugates for tumor microenvironment dependent drug delivery. J. Mater. Chem. B 2020, 8, 5745–5755. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, W.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Mechanical strong stretchable conductive multi-stimuli-responsive nanocomposite double network hydrogel as biosensor and actuator. J. Biomater. Sci. Polym. Ed. 2020, 31, 1770–1792. [Google Scholar] [CrossRef]
- Noda, I.; Ozaki, Y. Two-Dimensional Correlation Spectroscopy—Applications in Vibrational and Optical Spectroscopy; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Park, Y.; Noda, I.; Jung, Y.M. Two-dimensional correlation spectroscopy in polymer study. Front. Chem. 2015, 3, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Hu, J.; Tang, H.; Wu, P. Spectral interpretation of thermally irreversible recovery of poly (N-isopropylacrylamide-co-acrylic acid) hydrogel. Phys. Chem. Chem. Phys. 2011, 13, 5061–5067. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Hwang, M.; Kim, M.; Park, E.; Noda, I.; Jung, Y.M. Characterization of the Phase Transition Mechanism of P (NiPAAm-co-AAc) Copolymer Hydrogel Using 2D Correlation IR Spectroscopy. Spectrochim. Acta Part A 2021, 252, 119525. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.M.; Shin, H.S.; Czarnik-Matusewicz, B.; Noda, I.; Bin Kim, S. Characterization of Transition Temperatures of a Langmuir—Blodgett Film of Poly (tert-butyl Methacrylate) by Two-Dimensional Correlation Spectroscopy and Principal Component Analysis. Appl. Spectrosc. 2002, 56, 1568–1574. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.; Kim, M.; Chung, H.-j.; Woo, A.-h.; Noda, I.; Jung, Y.-m. The Study of pH Effects on Phase Transition of Multi-Stimuli Responsive P(NiPAAm-co-AAc) Hydrogel Using 2D-COS. Polymers 2021, 13, 1447. https://doi.org/10.3390/polym13091447
Park Y, Kim M, Chung H-j, Woo A-h, Noda I, Jung Y-m. The Study of pH Effects on Phase Transition of Multi-Stimuli Responsive P(NiPAAm-co-AAc) Hydrogel Using 2D-COS. Polymers. 2021; 13(9):1447. https://doi.org/10.3390/polym13091447
Chicago/Turabian StylePark, Yeonju, Minkyoung Kim, Hae-jin Chung, Ah-hyun Woo, Isao Noda, and Young-mee Jung. 2021. "The Study of pH Effects on Phase Transition of Multi-Stimuli Responsive P(NiPAAm-co-AAc) Hydrogel Using 2D-COS" Polymers 13, no. 9: 1447. https://doi.org/10.3390/polym13091447
APA StylePark, Y., Kim, M., Chung, H. -j., Woo, A. -h., Noda, I., & Jung, Y. -m. (2021). The Study of pH Effects on Phase Transition of Multi-Stimuli Responsive P(NiPAAm-co-AAc) Hydrogel Using 2D-COS. Polymers, 13(9), 1447. https://doi.org/10.3390/polym13091447