H2S-Sensing Studies Using Interdigitated Electrode with Spin-Coated Carbon Aerogel-Polyaniline Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CA
2.2. Preparation of Carbon Aerogel-Based Polyaniline Composites
2.3. Preparation of PANI and PANI/CA Sensor
2.4. Gas Sensing
3. Results
 where x + y = 1, when x = 1, y = 0 for the fully reduced polymer (so-called leuco-emeraldine); x = 0, y = 1 for the fully oxidised polymer (so-called pernigraniline); and x = 0.5, y = 0.5 for the half-oxidised polymer (emeraldine). CA was fabricated by high-temperature carbonisation of as-prepared phenolic resin. A specific feeding ratio of CA was incorporated into the PANI matrix to prepare the CA-based PANI composites.
where x + y = 1, when x = 1, y = 0 for the fully reduced polymer (so-called leuco-emeraldine); x = 0, y = 1 for the fully oxidised polymer (so-called pernigraniline); and x = 0.5, y = 0.5 for the half-oxidised polymer (emeraldine). CA was fabricated by high-temperature carbonisation of as-prepared phenolic resin. A specific feeding ratio of CA was incorporated into the PANI matrix to prepare the CA-based PANI composites.3.1. Characterisation
3.1.1. Polyaniline (PANI)
3.1.2. Carbon Aerogel (CA)
3.1.3. Composites
3.2. Morphological Observations of PANI, CA and Composite
3.3. H2S Gas-Sensing Performance
3.3.1. Gas-Sensing Mechanism
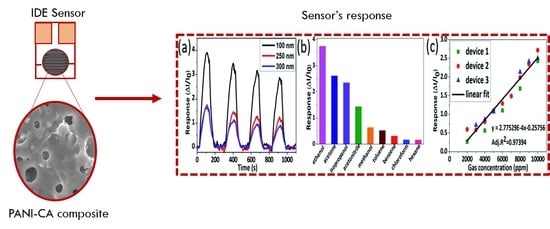
3.3.2. Sensor’s Response and Sensitivity
3.3.3. Response/Recovery Time
3.3.4. Repeatability
3.3.5. Selectivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, F.I.M.; Awwad, F.; Greish, Y.E.; Mahmud, S.T. Hydrogen sulphide (H2S) gas sensor-A Reivew. IEEE 2016, 19, 2394–2407. [Google Scholar]
- Khan, M.A.H.; Rao, M.V.; Li, Q. Recent Advances in Electrochemical sensors for detecting toxic gases: NO2, SO2 and H2S. Sensors 2019, 19, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, Y. Current Trends in Sensors Based on Conducting Polymer Nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.; Qazi, F.; Hussain, Z.; Idrees, M.U.; Soomro, S.; Soomro, S. Recent trends in electrochemical detection of NH3, H2S and NOX gases. Int. J. Electrochem. Sci. 2017, 12, 1711–1733. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Ding, X.; Yu, X.; Yu, X. Enhanced ammonia sensitive properties and mechanism research of PANI modified with hydroxylated single-walled nanotubes. Mater. Chem. Phys. 2019, 226, 378–386. [Google Scholar] [CrossRef]
- Baker, C.O.; Huang, X.; Nelson, W.; Kaner, R.B. Polyaniline nanofibers: Broadening applications for conducting polymers. Chem. Soc. Rev. 2017, 46, 1510–1525. [Google Scholar] [CrossRef]
- Ajeel, K.I.; Kareem, Q.S. Synthesis and characteristics of Polyaniline (PANI) filled by Graphene (PANI/GA) nano-films. J. Phys. Conf. Ser. 2019, 1234, 012020. [Google Scholar] [CrossRef] [Green Version]
- Weng, C.J.; Chen, Y.L.; Chien, C.M.; Hsu, S.C.; Jhuo, Y.S.; Yeh, J.M.; Dai, C.F. Preparation of gold decorated SiO2@polyaniline core–shell microspheres and application as a sensor for ascorbic acid. Electrochim. Acta 2013, 95, 162–169. [Google Scholar] [CrossRef]
- Hsu, S.C.; Cheng, H.T.; Wu, P.X.; Weng, C.J.; Santiago, K.S.; Yeh, J.M. Electrochemical sensor constructed using a carbon paste electrode modified with mesoporous silica encapsulating PANI chains decorated with CNPs for detection of ascorbic acid. Electrochim. Acta 2017, 238, 246–256. [Google Scholar] [CrossRef]
- Bibi, A.; Hsu, S.C.; Ji, W.F.; Cho, Y.C.; Santiago, K.S.; Yeh, J.M. Comparative Studies of CPEs Modified with Distinctive Metal Nanoparticle-Decorated Electroactive Polyimide for the Detection of UA. Polymers 2021, 13, 252. [Google Scholar] [CrossRef]
- Agbor, N.; Petty, M.; Monkman, A. Polyaniline thin films for gas sensing. Sens. Actuators B Chem. 1995, 28, 173–179. [Google Scholar] [CrossRef]
- Padua, L.M.G.; Yeh, J.M.; Santiago, K.S. A Novel Application of Electroactive Polyimide Doped with Gold Nanoparticles: As a Chemiresistor Sensor for Hydrogen Sulfide Gas. Polymers 2019, 11, 1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bibi, A.; Huang, C.H.; Lan, Y.X.; Chen, K.Y.; Ji, W.F.; Yeh, J.M.; Santiago, K.S.J. Effect of Surface Morphology of Electro-spun EPAA Coatings on the H2S Sensing Performance of Corresponding Interdigitated Electrodes. J. Electrochem. Soc. 2020, 167, 117510. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef] [Green Version]
- Molapo, K.M.; Ndangili, P.M.; Ajayi, R.F.; Mbambisa, G.; Mailu, S.M.; Njomo, N.; Masikini, M.; Baker, P.; Iwuoha, E.I. Elec-tronics of conjugated polymers (I): Polyaniline. Int. J. Electrochem. Sci. 2012, 7, 11859–11875. [Google Scholar]
- Pekala, R.W.; KONG, F.M. A synthetic route to organic aerogels-mechanism, structure, and properties. J. Phys. Colloq. 1989, 24, C4–C33. [Google Scholar] [CrossRef]
- Fung, A.W.P.; Wang, Z.H.; Lu, K.; Dresselhaus, M.S.; Pekala, R.W. Characterization of carbon aerogels by transport meas-urements. J. Mater. Res. Bull. 2006, 412, 553–562. [Google Scholar]
- Hanzawa, Y.; Kaneko, K.; Yoshizawa, N.; Pekala, R.; Dresselhaus, M. The Pore Structure Determination of Carbon Aerogels. Adsorption 1998, 4, 187–195. [Google Scholar] [CrossRef]
- Hosoya, M.; Reynolds, G.; Dresselhaus, M.; Pekala, R. Photoconductivity of carbon aerogels. J. Mater. Res. 1993, 8, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.L.; Ying, T.Y.; Yiacoumi, S.; Tsouris, C.; Vittoratos, E.S. Electrosorption of Ions from Aqueous Solutions by Carbon Aerogel: An Electrical Double-Layer Model. Langmuir 2001, 17, 1961–1969. [Google Scholar] [CrossRef]
- Horikawa, T.; Hayashi, J.; Muroyama, K. Controllability of pore characteristics of resorcinol–formaldehyde carbon aerogel. Carbon 2004, 42, 1625–1633. [Google Scholar] [CrossRef]
- Saliger, R.; Fischer, U.; Herta, C.; Fricke, J. High surface area carbon aerogels for supercapacitors. J. Non-Cryst. Solids 1998, 225, 81–85. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Maldonado-Hódar, F.J.; Rivera-Utrilla, J.; Rodríguez-Castellón, E. Group 6 metal oxide-carbon aerogels. Their synthesis, characterization and catalytic activity in the skeletal isomerization of 1-butene. Appl. Catal. A Gen. 1999, 183, 345–356. [Google Scholar] [CrossRef]
- Bekyarova, E.; Kaneko, K. Structure and physical properties of tailor-made Ce, Zr-doped carbon aerogels. Adv. Mater. 2000, 12, 1625–1628. [Google Scholar]
- Fu, R.; Zheng, B.; Liu, J.; Dresselhaus, M.S.; Dresselhaus, G.; Satcher, J.H.; Baumann, T.F. The Fabrication and Characterization of Carbon Aerogels by Gelation and Supercritical Drying in Isopropanol. Adv. Funct. Mater. 2003, 13, 558–562. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef]
- Wei, Y.Z.; Fang, B.; Iwasa, S.; Kumagai, M. A novel electrode material for electric double-layer capacitors. J. Power Sources 2005, 141, 386–391. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Shen, J.; Deng, Z.; Lai, Z.; Zhou, B.; Attia, S.; Chen, L. The investigation of the adsorption character of carbon aerogels. Nano Struct. Mater. 1999, 11, 375–381. [Google Scholar] [CrossRef]
- Li, F.; Ahmad, A.; Xie, L.J.; Sun, G.; Kong, Q.Q.; Su, F.Y.; Ma, Y.Y.; Chao, Y.; Guo, X.G.; Wei, X.X.; et al. Phos-phorous-modified porous carbon aerogel microspheres as high volumetric energy density electrode for supercapacitor. Electrochim. Acta 2019, 318, 151–160. [Google Scholar] [CrossRef]
- Virji, S.; Fowler, J.D.; Baker, C.O.; Huang, J.; Kaner, R.B.; Weiller, B.H. Polyaniline Nanofiber Composites with Metal Salts: Chemical Sensors for Hydrogen Sulfide. Small 2005, 1, 624–627. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, X.; Wu, X.; Cui, H.; Chen, D. Investigation of Gas-Sensing Property of Acid-Deposited Polyaniline Thin-Film Sensors for Detecting H2S and SO2. Sensors 2016, 16, 1889. [Google Scholar] [CrossRef] [Green Version]
- Crowley, K.; Morrin, A.; Shepherd, R.L.; in het Panhuis, M.; Wallace, G.G.; Smyth, M.R.; Killard, A.J. Fabrication of Polyani-line-Based Gas Sensors Using Piezoelectric Inkjet and Screen Printing for the Detection of Hydrogen Sulfide. IEEE Sens. J. 2010, 10, 1419–1426. [Google Scholar] [CrossRef] [Green Version]
- Mekki, A.; Joshi, N.; Singh, A.; Salmi, Z.; Jha, P.; Decorse, P.; Lau-Truong, S.; Mahmoud, R.; Chehimi, M.M.; Aswal, D.K.; et al. H2S sensing using in situ photo-polymerized polyaniline–silver nanocomposite films on flexible substrates. Org. Electron. 2014, 15, 71–81. [Google Scholar] [CrossRef]
- Gaikwad, G.; Patil, P.; Patil, D.; Naik, J. Synthesis and evaluation of gas sensing properties of PANI based graphene oxide nano-composites. Mater. Sci. Eng. B 2017, 218, 14–22. [Google Scholar] [CrossRef]
- Patil, L.; Pathan, I.; Suryawanshi, D.; Bari, A.; Rane, D. Spray Pyrolyzed ZnSnO3 Nanostructured Thin Films for Hydrogen Sensing. Procedia Mater. Sci. 2014, 6, 1557–1565. [Google Scholar] [CrossRef] [Green Version]
- Macagnano, A.; Perri, V.; Zampetti, E.; Bearzotti, A.; De Cesare, F. Humidity effects on a novel eco-friendly chemosensor based on electrospun PANi/PHB nanofibers. Sens. Actuators B Chem. 2016, 232, 16–27. [Google Scholar] [CrossRef]
- Li, Z.F.; Blumb, F.D.; Bertinoc, M.F.; Kim, C.S. Understanding the response of nanostructured polyaniline gas sensors. Sens. Actuators B Chem. 2013, 183, 419–427. [Google Scholar] [CrossRef]
- Abdelkader, R.; Amine, H.; Mohammed, B. Thermally Stable Forms of Pure Polyaniline Catalyzed by an Acid-Exchanged Montmorillonite Clay Called Maghnite-H+ as an Effective Catalyst. Int. J. Polym. Sci. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Naar, N.; Lamouri, S.; Jeacomine, I.; Pron, A.; Rinaudo, M. A Comprehensive Study and Characterization of Colloidal Emerald-ine-Base. J. Macromol. Sci. Part A Pure Appl. Chem. 2012, 49, 897–905. [Google Scholar] [CrossRef]
- Li, W.C.; Lu, A.H.; Guo, S.C. Characterization of the microstructures of organic and carbon aerogels based upon mixed cresol–formaldehyde. Carbon 2001, 39, 1989–1994. [Google Scholar] [CrossRef]
- Yan, M.F.; Zhang, L.H.; He, R.; Liu, Z.F. Synthesis and characterization of carbon aerogels with different catalysts. J. Porous Mater. 2015, 22, 699–703. [Google Scholar] [CrossRef]
- Macias, C.; Rasines, G.; García, T.E.; Zafra, M.C.; LaVela, P.; Tirado, J.L.; Ania, C.O. Synthesis of Porous and Mechanically Compliant Carbon Aerogels Using Conductive and Structural Additives. Gels 2016, 2, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tevlek, A.; Atya, A.M.; Almemar, M.; Duman, M.; Gokcen, D.; Ganin, A.Y.; Yiu, H.P.; Aydin, H.M. Synthesis of Conductive Carbon Aerogels Decorated with β-Tricalcium Phosphate Nanocrystallites. Sci. Rep. 2020, 10, 5758. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.J.; Lin, Y.J.; Chen, H.J.; Lin, Y.F.; Chuan Ho, K. A pt-free pristine monolithic carbon aerogel counter electrode for dye-sensitized solar cells: Up to 20% under dim light illumination. Nanoscale 2019, 11, 12507–12516. [Google Scholar] [CrossRef] [PubMed]
- Hebalkar, N.; Arabale, G.; Sainkar, S.R.; Pradhan, S.D.; Mulla, I.S.; Vijayamohanan, K.; Ayyub, P.; Kulkarni, S.K. Study of correlation of structural and surface properties with electrochemical behaviour in carbon aerogels. J. Mater. Sci. 2005, 40, 3777–3782. [Google Scholar] [CrossRef]
- Mousavi, S.; Kang, K.; Park, J.; Park, I. A room temperature hydrogen sulfide gas sensor based on electrospun polyaniline–polyethylene oxide nanofibers directly written on flexible substrates. RSC Adv. 2016, 6, 104131–104138. [Google Scholar] [CrossRef]













| Sensor | RH % | Sensitivity (ppm−1) | SD | R2 |
|---|---|---|---|---|
| PANI | 60% | 0.2293 | 0.007 | 0.9766 |
| PANI | 80% | 0.107 | 0.008 | 0.9943 |
| PANI-CA-1 | 60% | 0.3992 | 0.01 | 0.9786 |
| PANI-CA-1 | 80% | 0.1952 | 0.001 | 0.9922 |
| PANI-CA-3 | 60% | 0.4096 | 0.008 | 0.9887 |
| PANI-CA-3 | 80% | 0.2042 | 0.007 | 0.9915 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibi, A.; Rubio, Y.R.M.; Santiago, K.S.; Jia, H.-W.; Ahmed, M.M.M.; Lin, Y.-F.; Yeh, J.-M. H2S-Sensing Studies Using Interdigitated Electrode with Spin-Coated Carbon Aerogel-Polyaniline Composites. Polymers 2021, 13, 1457. https://doi.org/10.3390/polym13091457
Bibi A, Rubio YRM, Santiago KS, Jia H-W, Ahmed MMM, Lin Y-F, Yeh J-M. H2S-Sensing Studies Using Interdigitated Electrode with Spin-Coated Carbon Aerogel-Polyaniline Composites. Polymers. 2021; 13(9):1457. https://doi.org/10.3390/polym13091457
Chicago/Turabian StyleBibi, Aamna, Yuola Rose M. Rubio, Karen S. Santiago, His-Wei Jia, Mahmoud M. M. Ahmed, Yi-Feng Lin, and Jui-Ming Yeh. 2021. "H2S-Sensing Studies Using Interdigitated Electrode with Spin-Coated Carbon Aerogel-Polyaniline Composites" Polymers 13, no. 9: 1457. https://doi.org/10.3390/polym13091457
APA StyleBibi, A., Rubio, Y. R. M., Santiago, K. S., Jia, H. -W., Ahmed, M. M. M., Lin, Y. -F., & Yeh, J. -M. (2021). H2S-Sensing Studies Using Interdigitated Electrode with Spin-Coated Carbon Aerogel-Polyaniline Composites. Polymers, 13(9), 1457. https://doi.org/10.3390/polym13091457