UV-Curable Bio-Based Polymers Derived from Industrial Pulp and Paper Processes
Abstract
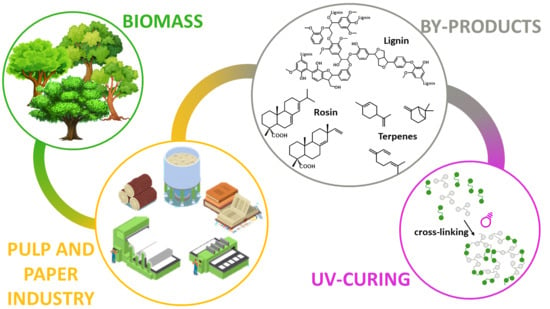
:1. Introduction
1.1. Lignin and Derivatives
- Kraft lignin (KL), which is a by-product of the paper and pulp industry after lignocellulose has been treated at an elevated temperature (170 °C) and pressure. KL is becoming the added value product of this industry, transforming the entire process into a bio-refinery system.
- Lignosulfonates (LS), which are derived from the sulfite process, are obtained through a neutral or acidic treatment/cooking. Sulfite pulping is the second most commercially-used process, and it produces about 7 million tons of lignin annually.
- Soda lignin (SL), which is generated as a co-product from flax, straw, and/or non-wood fibers, using anthraquinone as a catalyst. However, SL has a low production capacity, due to the annual variability of the feedstock.
- Organosolv lignin (OL), which is obtained from pulp by means of an organic solvent treatment. In this process, the lignin is obtained using solvents, using neither acidic or alkaline conditions, and it is an alternative process to the pulping technology. The extraction of lignin with an organic solvent mainly involves breaking down the α-aryl ether bonds. OL is usually less contaminated than other types of technical lignins, and with recovery of the solvent it is a closed process.
- Steam-explosion lignin (SEL), which is derived from a steam process. The process involves the impregnation of lignin with steam at high temperature and pressure. This leads to an increase in the reactivity of the substrate, allowing more enzyme access and digestibility in order to separate the components.
- Hydrogenolysis, which is a pyrolysis process that results in the formation of small fragments in the presence of hydrogen. Cleavage refers to the C–C bond [17]. Typical temperatures of around 300–600 °C are used. Catalysts, such as Ru/C, Pd/C or Pt/C, are used to improve the final yield of the product, and bimetallic systems have also been explored. An appropriate choice of the catalyst leads to a high degree of depolymerization and a high product yield. The hydrogenolysis of the C–C bond is catalyst dependent, as shown in Figure 4 [8].
- Oxidation, whereby lignin is generally oxidized with nitrobenzene, hydrogen peroxide or a metal oxide. Some methods use heterogeneous catalysts, such as metal or surface-supported metal, Pt, Pd, Re, Ti, Ni and Cu. Homogeneous catalysts include meta and organic compounds; ionic liquid has also been used as an alternative catalytic system [1].
1.2. Rosins and Its Derivatives
1.3. Terpenes
2. UV-Curable Monomers from the Pulp and Paper Industry
2.1. The Photopolymerization of Lignin and Its Derivatives
2.2. The Photopolymerization of Rosin
2.3. The Photopolymerization of Terpenes
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Upton, B.M.; Kasko, A.M. Strategies for the Conversion of Lignin to High-Value Polymeric Materials: Review and Perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef]
- Torres, L.A.Z.; Woiciechowski, A.L.; Tanobe, V.O.D.A.; Karp, S.G.; Lorenci, L.C.G.; Faulds, C.; Soccol, C.R. Lignin as a potential source of high-added value compounds: A review. J. Clean. Prod. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Haq, I.; Mazumder, P.; Kalamdhad, A.S. Recent advances in removal of lignin from paper industry wastewater and its industrial applications—A review. Bioresour. Technol. 2020, 312, 123636. [Google Scholar] [CrossRef]
- Alén, R. Pulp Mills and Wood-Based Biorefineries; Elsevier BV: Amsterdam, The Netherlands, 2015; ISBN 9780444634535. [Google Scholar]
- Bajpai, P. Chemicals and Fuels from Residuals and Spent Pulping Liquors. Pulp Pap. Ind. 2017, 205–220. [Google Scholar] [CrossRef]
- Bajpai, P. Biermann’s Handbook of Pulp and Paper. Pulping Fundam. 2018, 295–351. [Google Scholar] [CrossRef]
- Cheng, K. Industrial Scale Lignin Recovery from Pulping Liquors; Elsevier BV: Amsterdam, The Netherlands, 2021; pp. 123–145. [Google Scholar]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandini, A.; Lacerda, T.M. From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- John, G.; Nagarajan, S.; Vemula, P.K.; Silverman, J.R.; Pillai, C. Natural monomers: A mine for functional and sustainable materials—Occurrence, chemical modification and polymerization. Prog. Polym. Sci. 2019, 92, 158–209. [Google Scholar] [CrossRef]
- Wilbon, P.A.; Chu, F.; Tang, C. Progress in Renewable Polymers from Natural Terpenes, Terpenoids, and Rosin. Macromol. Rapid Commun. 2013, 34, 8–37. [Google Scholar] [CrossRef]
- Wan, J.; Zhao, J.; Zhang, X.; Fan, H.; Zhang, J.; Hu, D.; Jin, P.; Wang, D.-Y. Epoxy thermosets and materials derived from bio-based monomeric phenols: Transformations and performances. Prog. Polym. Sci. 2020, 108, 101287. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.-P. Biobased Thermosetting Epoxy: Present and Future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Epoxy thermosets from model mixtures of the lignin-to-vanillin process. Green Chem. 2016, 18, 712–725. [Google Scholar] [CrossRef]
- Jawerth, M.; Lawoko, M.; Lundmark, S.; Perez-Berumen, C.; Johansson, M. Allylation of a lignin model phenol: A highly selective reaction under benign conditions towards a new thermoset resin platform. RSC Adv. 2016, 6, 96281–96288. [Google Scholar] [CrossRef] [Green Version]
- Jaswal, S.; Gaur, B. Structure-property correlation study of bio-based multifunctional vinyl ester resin in presence of methacrylated lignin model compounds. Polym. Sci. Ser. B 2015, 57, 417–433. [Google Scholar] [CrossRef]
- Sangermano, M.; Roppolo, I.; Messori, M. UV-cured functional coatings. In Photocured Materials; Tiwari, A., Polykarpov, A., Eds.; Royal Society of Chemistry: London, UK, 2014; p. 121. [Google Scholar]
- Belgacem, M.N.; Gandini, A. Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 0080560512. [Google Scholar]
- Liao, J.J.; Latif, N.H.A.; Trache, D.; Brosse, N.; Hussin, M.H. Current advancement on the isolation, characterization and application of lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.; Liu, Y.; Ruan, X.; Tsang, D.C.; Hunt, A.J.; Ok, Y.S.; Song, H.; Zhang, S. Lignin valorization for the production of renewable chemicals: State-of-the-art review and future prospects. Bioresour. Technol. 2018, 269, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Lora, J.H.; Glasser, W.G. Recent Industrial Applications of Lignin: A Sustainable Alternative to Nonrenewable Materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.; Chu, F. Wood-Derived Functional Polymeric Materials. Adv. Mater. 2020, 2001135, e2001135. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin Production from Lignin and Its Use as a Renewable Chemical. ACS Sustain. Chem. Eng. 2016, 4, 35–46. [Google Scholar] [CrossRef]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevin, B. Vanillin, a promising biobased building-block for monomer synthesis. Green Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. From Lignin-derived Aromatic Compounds to Novel Biobased Polymers. Macromol. Rapid Commun. 2016, 37, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Pion, F.; Ducrot, P.-H.; Allais, F. Renewable Alternating Aliphatic-Aromatic Copolyesters Derived from Biobased Ferulic Acid, Diols, and Diacids: Sustainable Polymers with Tunable Thermal Properties. Macromol. Chem. Phys. 2014, 215, 431–439. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Patto, M.C.V.; Bronze, M.D.R. Relevance, structure and analysis of ferulic acid in maize cell walls. Food Chem. 2018, 246, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.E. Ferulic Acid: An Antioxidant Found Naturally in Plant Cell Walls and Feruloyl Esterases Involved in its Release and Their Applications. Crit. Rev. Biotechnol. 2004, 24, 59–83. [Google Scholar] [CrossRef]
- Li, M.; Jia, Z.; Wan, G.; Wang, S.; Min, D. Enhancing isolation of p-coumaric and ferulic acids from sugarcane bagasse by sequential hydrolysis. Chem. Pap. 2020, 74, 499–507. [Google Scholar] [CrossRef]
- Araujo, E.D.S.; Mota, G.D.S.; Lorenço, M.S.; Zidanes, U.L.; da Silva, L.R.; Silva, E.P.; Ferreira, V.R.F.; Cardoso, M.D.G.; Mori, F.A. Characterisation and valorisation of the bark of Myrcia eximia DC. trees from the Amazon rainforest as a source of phenolic compounds. Holzforschung 2020, 74, 989–998. [Google Scholar] [CrossRef]
- Ou, S.; Luo, Y.; Huang, C.; Jackson, M. Production of coumaric acid from sugarcane bagasse. Innov. Food Sci. Emerg. Technol. 2009, 10, 253–259. [Google Scholar] [CrossRef]
- Duan, J.; Huo, X.; Du, W.; Liang, J.; Wang, D.; Yang, S. Biodegradation of kraft lignin by a newly isolated anaerobic bacterial strain, Acetoanaerobium sp. WJDL-Y2. Lett. Appl. Microbiol. 2016, 62, 55–62. [Google Scholar] [CrossRef]
- Williamson, J.J.; Bahrin, N.; Hardiman, E.M.; Bugg, T.D.H. Production of Substituted Styrene Bioproducts from Lignin and Lignocellulose Using Engineered Pseudomonas putida KT2440. Biotechnol. J. 2020, 15, e1900571. [Google Scholar] [CrossRef]
- Zhao, C.-H.; Zhang, R.-K.; Qiao, B.; Li, B.-Z.; Yuan, Y.-J. Engineering budding yeast for the production of coumarins from lignin. Biochem. Eng. J. 2020, 160, 107634. [Google Scholar] [CrossRef]
- Sibhatu, H.K.; Jabasingh, S.A.; Yimam, A.; Ahmed, S. Ferulic acid production from brewery spent grains, an agro-industrial waste. LWT 2021, 135, 110009. [Google Scholar] [CrossRef]
- Kugler, S.; Ossowicz, P.; Malarczyk-Matusiak, K.; Wierzbicka, E. Advances in Rosin-Based Chemicals: The Latest Recipes, Applications and Future Trends. Molecules 2019, 24, 1651. [Google Scholar] [CrossRef] [Green Version]
- Silvestre, A.J.; Gandini, A. Rosin: Major Sources, Properties and Applications. Monom. Polym. Compos. Renew. Resour. 2008, 67–88. [Google Scholar] [CrossRef]
- Grand View Research Rosin Market Size, Share & Trends Analysis Report by Product, by Application, Regional Outlook, Competitive Strategies, and Segment Forecasts, 2019 to 2025. Available online: https://www.grandviewresearch.com/industry-analysis/rosin-market (accessed on 16 November 2020).
- Silvestre, A.J.; Gandini, A. Terpenes: Major Sources, Properties and Applications. Monom. Polym. Compos. Renew. Resour. 2008, 17–38. [Google Scholar] [CrossRef]
- Tsujimura, M.; Tsuji, M.; Kofujita, H.; Ohira, T. New separation method for terpenoids in byproducts discharged during sugi wood-drying process, and purification of ferruginol. J. Wood Sci. 2015, 61, 308–315. [Google Scholar] [CrossRef]
- Tsujimura, M.; Goto, M.; Tsuji, M.; Yamaji, Y.; Ashitani, T.; Kimura, K.-I.; Ohira, T.; Kofujita, H. Isolation of diterpenoids from sugi wood-drying byproducts and their bioactivities. J. Wood Sci. 2019, 65, 19. [Google Scholar] [CrossRef] [Green Version]
- Hatakeyama, H.; Hirose, S.; Hatakeyama, T. High-Performance Polymers from Lignin Degradation Products. In Lignin; Glasser, G.W., Sarkanen, S., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1989; Volume 397, pp. 15–205. ISBN 9780841216310. [Google Scholar]
- Gandini, A.; Belgacem, N.M. Recent advances in the elaboration of polymeric materials derived from biomass components. Polym. Int. 1998, 47, 267–276. [Google Scholar] [CrossRef]
- Gandini, A.; Belgacem, M.N. Recent Contributions to the Realm of Polymers from Renewable Resources. ACS Symp. Ser. 2007, 954, 48–60. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Blayo, A.; Gandini, A. Organosolv lignin as a filler in inks, varnishes and paints. Ind. Crop. Prod. 2003, 18, 145–153. [Google Scholar] [CrossRef]
- Doherty, W.; Halley, P.; Edye, L.; Rogers, D.; Cardona, F.; Park, Y.; Woo, T. Studies on polymers and composites from lignin and fiber derived from sugar cane. Polym. Adv. Technol. 2007, 18, 673–678. [Google Scholar] [CrossRef]
- Park, Y.; Doherty, W.; Halley, P.J. Developing lignin-based resin coatings and composites. Ind. Crop. Prod. 2008, 27, 163–167. [Google Scholar] [CrossRef]
- Cazacu, G.; Capraru, M.; Popa, V.I. Advances Concerning Lignin Utilization in New Materials. In Advances in Natural Polymers; Sabu, T., Visakh, P.M., Aji, P.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 255–312. [Google Scholar]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Alsulami, Q.A.; Albukhari, S.M.; Hussein, M.A.; Tay, G.S.; Rozman, H.D. Biodegradable lignin as a reactive raw material in UV curable systems. Polym. Technol. Mater. 2020, 59, 1387–1406. [Google Scholar] [CrossRef]
- Rozman, H.D.; Koay, E.L.; Tay, G.S. Preliminary study on the utilization of lignin as filler in ultra-violet (UV) curable system. J. Appl. Polym. Sci. 2010, 120, 2527–2533. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Hao, N.; Ragauskas, A.J. Stereolithography 3D Printing of Lignin-Reinforced Composites with Enhanced Mechanical Properties. ACS Omega 2019, 4, 20197–20204. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, F.; Mohan, D.; Sajab, M.S.; Bakarudin, S.B.; Kaco, H. Evaluation of the Compatibility of Organosolv Lignin-Graphene Nanoplatelets with Photo-Curable Polyurethane in Stereolithography 3D Printing. Polymers 2019, 11, 1544. [Google Scholar] [CrossRef] [Green Version]
- Chao, J.L.; Chu, F.Q.; Xu, C.L.; Wang, H.; Liu, Y.X. The Property of Waterborne UV-Curable Polyurethane Based on Alkali Lignin of Wheat Straw. Adv. Mater. Res. 2013, 834-836, 199–202. [Google Scholar] [CrossRef]
- Wang, C.; Venditti, R.A. UV Cross-Linkable Lignin Thermoplastic Graft Copolymers. ACS Sustain. Chem. Eng. 2015, 3, 1839–1845. [Google Scholar] [CrossRef]
- Hajirahimkhan, S.; Ragogna, P.J.; Xu, C. Methacrylation of kraft lignin for UV-curable coatings: Process optimization using response surface methodology. Biomass Bioenergy 2019, 120, 332–338. [Google Scholar] [CrossRef]
- Hajirahimkhan, S.; Xu, C.C.; Ragogna, P.J. Ultraviolet Curable Coatings of Modified Lignin. ACS Sustain. Chem. Eng. 2018, 6, 14685–14694. [Google Scholar] [CrossRef]
- Yan, R.; Yang, D.; Zhang, N.; Zhao, Q.; Liu, B.; Xiang, W.; Sun, Z.; Xu, R.; Zhang, M.; Hu, W. Performance of UV curable lignin based epoxy acrylate coatings. Prog. Org. Coat. 2018, 116, 83–89. [Google Scholar] [CrossRef]
- Yan, R.; Liu, Y.; Liu, B.; Zhang, Y.; Zhao, Q.; Sun, Z.; Hu, W.; Zhang, N. Improved performance of dual-cured organosolv lignin-based epoxy acrylate coatings. Compos. Commun. 2018, 10, 52–56. [Google Scholar] [CrossRef]
- Sutton, J.T.; Rajan, K.; Harper, D.P.; Chmely, S.C. Lignin-Containing Photoactive Resins for 3D Printing by Stereolithography. ACS Appl. Mater. Interfaces 2018, 10, 36456–36463. [Google Scholar] [CrossRef]
- Ding, R.; Du, Y.; Goncalves, R.B.; Francis, L.F.; Reineke, T.M. Sustainable near UV-curable acrylates based on natural phenolics for stereolithography 3D printing. Polym. Chem. 2019, 10, 1067–1077. [Google Scholar] [CrossRef]
- Bassett, A.W.; Honnig, A.E.; Breyta, C.M.; Dunn, I.C.; la Scala, J.J.; Stanzione, I.J.F. Vanillin-Based Resin for Additive Manufacturing. ACS Sustain. Chem. Eng. 2020, 8, 5626–5635. [Google Scholar] [CrossRef]
- Maturi, M.; Pulignani, C.; Locatelli, E.; Buratti, V.V.; Tortorella, S.; Sambri, L.; Franchini, M.C. Phosphorescent bio-based resin for digital light processing (DLP) 3D-printing. Green Chem. 2020, 22, 6212–6224. [Google Scholar] [CrossRef]
- Navaruckiene, A.; Skliutas, E.; Kasetaite, S.; Rekštytė, S.; Raudoniene, V.; Bridziuviene, D.; Malinauskas, M.; Ostrauskaite, J. Vanillin Acrylate-Based Resins for Optical 3D Printing. Polymers 2020, 12, 397. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.C.; Lima, M.S.; Sousa, A.F.; Silvestre, A.J.; Coelho, J.F.J.; Serra, A.C. Cinnamic acid derivatives as promising building blocks for advanced polymers: Synthesis, properties and applications. Polym. Chem. 2019, 10, 1696–1723. [Google Scholar] [CrossRef]
- Nagata, M.; Hizakae, S. Synthesis and Characterization of Photocrosslinkable Biodegradable Polymers Derived from 4-Hydroxycinnamic Acid. Macromol. Biosci. 2003, 3, 412–419. [Google Scholar] [CrossRef]
- Ménard, R.; Caillol, S.; Allais, F. Ferulic acid-based renewable esters and amides-containing epoxy thermosets from wheat bran and beetroot pulp: Chemo-enzymatic synthesis and thermo-mechanical properties characterization. Ind. Crop. Prod. 2017, 95, 83–95. [Google Scholar] [CrossRef]
- Xin, J.; Zhang, P.; Huang, K.; Zhang, J. Study of green epoxy resins derived from renewable cinnamic acid and dipentene: Synthesis, curing and properties. RSC Adv. 2014, 4, 8525–8532. [Google Scholar] [CrossRef]
- Hughes, T.; Simon, G.P.; Saito, K. Chemistries and capabilities of photo-formable and photoreversible crosslinked polymer networks. Mater. Horiz. 2019, 6, 1762–1773. [Google Scholar] [CrossRef]
- Teramoto, N.; Shibata, M. Synthesis and photocuring of cinnamoyl trehalose esters. Polym. Adv. Technol. 2007, 18, 971–977. [Google Scholar] [CrossRef]
- Yano, S.; Iwase, T.; Teramoto, N.; Shimasaki, T.; Shibata, M. Synthesis, thermal properties and cell-compatibility of photocrosslinked cinnamoyl-modified hydroxypropyl cellulose. Carbohydr. Polym. 2018, 184, 418–426. [Google Scholar] [CrossRef]
- Castillo, E.A.; Miura, H.; Hasegawa, M.; Ogawa, T. Synthesis of novel polyamides starting from ferulic acid dimer derivative. Des. Monom. Polym. 2004, 7, 711–725. [Google Scholar] [CrossRef] [Green Version]
- Tunc, D.; le Coz, C.; Alexandre, M.; Desbois, P.; le Comte, P.; Carlotti, S. Reversible Cross-Linking of Aliphatic Polyamides Bearing Thermo- and Photoresponsive Cinnamoyl Moieties. Macromolecules 2014, 47, 8247–8254. [Google Scholar] [CrossRef]
- Hu, X.; Chen, X.; Cheng, H.; Jing, X. Cinnamate-functionalized poly(ester-carbonate): Synthesis and its UV irradiation-induced photo-crosslinking. J. Polym. Sci. Part A Polym. Chem. 2008, 47, 161–169. [Google Scholar] [CrossRef]
- Nagata, M.; Sato, Y. Biodegradable elastic photocured polyesters based on adipic acid, 4-hydroxycinnamic acid and poly(ε-caprolactone) diols. Polymers 2004, 45, 87–93. [Google Scholar] [CrossRef]
- Kim, D.M.; Yu, S.H.; Lee, J.Y.; Min, K.D.; Hun, Y.S.; Young, L.J. Synthesis and Characterization of Thermally Stable Photocurable Polymer with Cyclohexane Moiety. J. Nanosci. Nanotechnol. 2016, 16, 2641–2645. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chen, Y.F.; Zhong, Z.; Lu, F.; Du, Y.; Liu, L.; Huang, Y. Preparation of the flexible soybean oil-based material via [2 + 2] cycloaddition photo-polymerization. J. Appl. Polym. Sci. 2021, 138. [Google Scholar] [CrossRef]
- Shibata, M.; Sugane, K.; Yanagisawa, Y. Biobased polymer networks by the thiol-ene photopolymerization of allylated p-coumaric and caffeic acids. Polym. J. 2019, 51, 461–470. [Google Scholar] [CrossRef]
- Pezzana, L.; Mousa, M.; Malmström, E.; Johansson, M.; Sangermano, M. Bio-based monomers for UV-curable coatings: Allylation of ferulic acid and investigation of photocured thiolene network. Prog. Org. Coat. 2021, 150, 105986. [Google Scholar] [CrossRef]
- Chen, G.-F. Developments in the field of rosin chemistry and its implications in coatings. Prog. Org. Coat. 1992, 20, 139–167. [Google Scholar] [CrossRef]
- Lee, J.S.; Hong, S.I. Synthesis of acrylic rosin derivatives and application as negative photoresist. Eur. Polym. J. 2002, 38, 387–392. [Google Scholar] [CrossRef]
- Do, H.-S.; Park, J.-H.; Kim, H.-J. Synthesis and characteristics of photoactive-hydrogenated rosin epoxy methacrylate for pressure sensitive adhesives. J. Appl. Polym. Sci. 2009, 111, 1172–1176. [Google Scholar] [CrossRef]
- Do, H.-S.; Park, J.-H.; Kim, H.-J. UV-curing behavior and adhesion performance of polymeric photoinitiators blended with hydrogenated rosin epoxy methacrylate for UV-crosslinkable acrylic pressure sensitive adhesives. Eur. Polym. J. 2008, 44, 3871–3882. [Google Scholar] [CrossRef]
- Ahn, B.K.; Sung, J.; Kim, N.; Kraft, S.; Sun, X.S. UV-curable pressure-sensitive adhesives derived from functionalized soybean oils and rosin ester. Polym. Int. 2012, 62, 1293–1301. [Google Scholar] [CrossRef]
- Liu, B.; Nie, J.; He, Y. From rosin to high adhesive polyurethane acrylate: Synthesis and properties. Int. J. Adhes. Adhes. 2016, 66, 99–103. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Z.; Gu, Y.; Chen, Y.; Bi, L. Synthesis of rosin allyl ester and its UV-curing characteristics. Polym. J. 2011, 43, 869–873. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Z.; Chen, Y.; Wang, J.; Xu, S.; Gu, Y. Synthesis of allyl acrylpimarate by microwave irradiation and phase-transfer catalytic reaction and its UV-curing reactions as a new monomer. Prog. Org. Coat. 2017, 109, 9–21. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Z.; Bi, L.; Chen, Y.; Wang, J.; Xu, S. Synthesis of a multifunctional hard monomer from rosin: The relationship of allyl structure in maleopimarate and UV-curing property. Sci. Rep. 2018, 8, 2399. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zhao, Z.; Bi, L.; Chen, Y.; Gu, Y.; Wang, J. Preparation and polymerization of isopimaric acid derivatives. J. Appl. Polym. Sci. 2019, 136, 1–6. [Google Scholar] [CrossRef]
- Lu, C.; Wang, C.; Yu, J.; Wang, J.; Chu, F. Two-Step 3 D-Printing Approach toward Sustainable, Repairable, Fluorescent Shape-Memory Thermosets Derived from Cellulose and Rosin. ChemSusChem 2019, 13, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Crivello, J.V.; Yang, B. Studies of synthesis and cationic photopolymerization of three isomeric monoterpene diepoxides. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 1881–1890. [Google Scholar] [CrossRef]
- Crivello, J.V.; Liu, S. Free Radical Induced Acceleration of Cationic Photopolymerization. Chem. Mater. 1998, 10, 3724–3731. [Google Scholar] [CrossRef]
- Crivello, J.V.; Liu, S.S. Synthesis and cationic photopolymerization of monomers based on nopol. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 1199–1209. [Google Scholar] [CrossRef]
- Crivello, J.V.; Liu, S. Photoinitiated cationic polymerization of epoxy alcohol monomers. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 389–401. [Google Scholar] [CrossRef]
- Park, H.J.; Ryu, C.Y.; Crivello, J.V. Photoinitiated cationic polymerization of limonene 1,2-oxide and α-pinene oxide. J. Polym. Sci. Part A Polym. Chem. 2012, 51, 109–117. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lalevée, J.; Gigmes, D.; Fouassier, J.P. Green Chemistry: Sunlight-Induced Cationic Polymerization of Renewable Epoxy Monomers under Air. Macromolecules 2010, 43, 1364–1370. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lalevée, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P. A Breakthrough toward Long Wavelength Cationic Photopolymerization: Initiating Systems Based on Violanthrone Derivatives and Silyl Radicals. Macromolecules 2011, 44, 8374–8379. [Google Scholar] [CrossRef]
- Lalevée, J.; Fouassier, J.P. Recent advances in sunlight induced polymerization: Role of new photoinitiating systems based on the silyl radical chemistry. Polym. Chem. 2011, 2, 1107–1113. [Google Scholar] [CrossRef]
- Claudino, M.; Mathevet, J.-M.; Johansson, M. Bringingd-limonene to the scene of bio-based thermoset coatings via free-radical thiol–ene chemistry: Macromonomer synthesis, UV-curing and thermo-mechanical characterization. Polym. Chem. 2014, 5, 3245–3260. [Google Scholar] [CrossRef] [Green Version]
- Breloy, L.; Ouarabi, C.A.; Brosseau, A.; Dubot, P.; Brezova, V.; Andaloussi, S.A.; Malval, J.-P.; Versace, D.-L. β-Carotene/Limonene Derivatives/Eugenol: Green Synthesis of Antibacterial Coatings under Visible-Light Exposure. ACS Sustain. Chem. Eng. 2019, 7, 19591–19604. [Google Scholar] [CrossRef]
- Stamm, A.; Tengdelius, M.; Schmidt, B.; Engström, J.; Syrén, P.-O.; Fogelström, L.; Malmström, E. Chemo-enzymatic pathways toward pinene-based renewable materials. Green Chem. 2019, 21, 2720–2731. [Google Scholar] [CrossRef] [Green Version]
- Weems, A.C.; Chiaie, K.R.D.; Worch, J.C.; Stubbs, C.J.; Dove, A.P. Terpene- and terpenoid-based polymeric resins for stereolithography 3D printing. Polym. Chem. 2019, 10, 5959–5966. [Google Scholar] [CrossRef] [Green Version]
- Weems, A.C.; Chiaie, K.R.D.; Yee, R.; Dove, A.P. Selective Reactivity of Myrcene for Vat Photopolymerization 3D Printing and Postfabrication Surface Modification. Biomacromolecules 2019, 21, 163–170. [Google Scholar] [CrossRef]
- Schimpf, V.; Asmacher, A.; Fuchs, A.; Stoll, K.; Bruchmann, B.; Mülhaupt, R. Low-Viscosity Limonene Dimethacrylate as a Bio-Based Alternative to Bisphenol A-Based Acrylic Monomers for Photocurable Thermosets and 3D Printing. Macromol. Mater. Eng. 2020, 305. [Google Scholar] [CrossRef]
- Ortiz, R.A.; Valdez, A.E.G.; Cruz, D.H.; Jiménez, G.N.; Jiménez, A.I.H.; Padilla, J.G.T.; Santos, R.G. Highly reactive novel biobased cycloaliphatic epoxy resins derived from nopol and a study of their cationic photopolymerization. J. Polym. Res. 2020, 27, 1–16. [Google Scholar] [CrossRef]
- Li, C.; Johansson, M.; Buijsen, P.; Dijkstra, G.; Sablong, R.J.; Koning, C.E. Limonene-derived polycarbonates as biobased UV-curable (powder) coating resins. Prog. Org. Coat. 2021, 151, 106073. [Google Scholar] [CrossRef]





















| Lignocellulosic Materials | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| Hardwood stems | 40–55 | 24–40 | 18–25 |
| Softwood stems | 45–50 | 25–35 | 25–35 |
| Nut shells | 25–30 | 25–30 | 30–40 |
| Corn cobs | 45 | 35 | 15 |
| Wheat straw | 30 | 50 | 15 |
| Rice straw | 32 | 24 | 18 |
| Leaves | 15–20 | 80–85 | 0 |
| Grasses | 25–40 | 25-50 | 10–30 |
| Switch grass | 31–32 | 35–50 | 20–25 |
| Sugarcane bagasse | 42 | 25 | 20 |
| Sweet sorghum | 45 | 27 | 21 |
| Cotton seed hairs | 80–95 | 5–20 | 0 |
| Coconut husk | 39 | 16 | 30 |
| Sorted refuse | 60 | 20 | 20 |
| Paper | 85–99 | 0 | 0–15 |
| Newspaper | 40–55 | 25–40 | 18–30 |
| Waste paper from chemical pulps | 60–70 | 10–20 | 5–10 |
| Primary wastewater solids | 8–15 | NA | 24–29 |
| Monolignol | Broadleaf Wood (%) | Conifer Wood (%) | Grass (%) |
|---|---|---|---|
| Sinapyl alcohol (S) | 50–75 | 0–1 | 25–50 |
| Coniferyl alcohol (G) | 25–50 | 90–95 | 25–50 |
| p-Coumaryl alcohol (H) | Trace | 0.5–3.4 | 10–25 |
| Phenolic Acid and Aldehydes | Lignin Fraction (% yield, w/w) | ||||
|---|---|---|---|---|---|
| OPF Lignin | Caligonum Monogoliacum Lignin | Tamarix spp. Lignin | Maize Stem Lignin | Rice Straw Lignin | |
| p-Hydroxybenzoic acid | 0.42 | 1.68 | 1.67 | 0.82 | 1.12 |
| p-Hydroxybenzaldehyde | 0.49 | 1.35 | 1.21 | 2.48 | 1.59 |
| Vanillic acid | 0.25 | 1.04 | 1.14 | 0.03 | 0.36 |
| Syringic acid | 0.84 | 2.16 | 1.77 | 1.28 | 1.82 |
| Vanillin | 1.02 | 17.96 | 18.12 | 10.49 | 15.49 |
| Syringaldehyde | 2.60 | 9.36 | 10.34 | 13.05 | 13.00 |
| p-Coumaric acid | N/A | 3.08 | 2.55 | 0.32 | 0.61 |
| Ferulic acid | 0.30 | 0.91 | 0.47 | 0.82 | 1.22 |
| Molar ratio (S:G:H) | 58:22:15 | 58:22:15 | 31:59:10 | N/A | N/A |
| Lignin Type | Maximum Amount (wt %) | Role of Lignin | Reference |
|---|---|---|---|
| Acell lignin | 20 | filler | Rozman [53] |
| Kraft lignin | 1 | filler | Zhang [54] |
| Organosolv lignin | 3 | filler | Ibrahim [55] |
| Alkali lignin (hydroxymethylated) | 12 | monomer | Chao [56] |
| Kraft lignin (copolymerized) | - | monomer | Wang [57] |
| Kraft lignin (methacrylated) | 30 | monomer | Hajirahimkhan [58] |
| Kraft lignin (methacrylated) | 31 | monomer | Hajirahimkhan [59] |
| Organosolv lignin (epoxy acrylate) | 25 | monomer | Yan [60] |
| Organosolv lignin (epoxy acrylate) | 25 | monomer | Yan [61] |
| Organosolv lignin (acrylate) | 15 | monomer | Sutton [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzana, L.; Malmström, E.; Johansson, M.; Sangermano, M. UV-Curable Bio-Based Polymers Derived from Industrial Pulp and Paper Processes. Polymers 2021, 13, 1530. https://doi.org/10.3390/polym13091530
Pezzana L, Malmström E, Johansson M, Sangermano M. UV-Curable Bio-Based Polymers Derived from Industrial Pulp and Paper Processes. Polymers. 2021; 13(9):1530. https://doi.org/10.3390/polym13091530
Chicago/Turabian StylePezzana, Lorenzo, Eva Malmström, Mats Johansson, and Marco Sangermano. 2021. "UV-Curable Bio-Based Polymers Derived from Industrial Pulp and Paper Processes" Polymers 13, no. 9: 1530. https://doi.org/10.3390/polym13091530
APA StylePezzana, L., Malmström, E., Johansson, M., & Sangermano, M. (2021). UV-Curable Bio-Based Polymers Derived from Industrial Pulp and Paper Processes. Polymers, 13(9), 1530. https://doi.org/10.3390/polym13091530