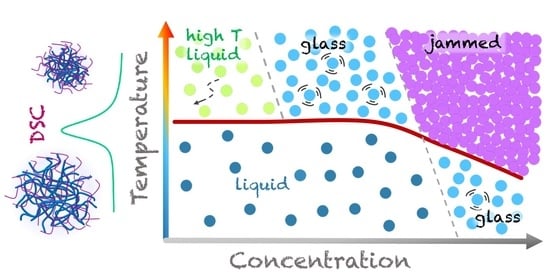
Thermal Behaviour of Microgels Composed of Interpenetrating Polymer Networks of Poly(N-isopropylacrylamide) and Poly(acrylic acid): A Calorimetric Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microgel Synthesis
2.3. Differential Scanning Calorimetric Measurements
2.4. Dynamic Light Scattering Measurements
3. Results and Discussion
3.1. Effect of Heating Rate
3.2. Effect of Weight Concentration
3.3. Effect of pH
3.4. Effect of PAAc Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandez-Nieves, A.; Wyss, H.M.; Mattsson, J.; Weitz, D. Microgel Suspensions: Fundamental and Applications; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and microgels: From model colloids to applications, recent developments, and future trends. Langmuir 2019, 35, 6231–6255. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, B.; Hu, Z. Phase Behavior of Thermally Responsive Microgel Colloids. Phys. Rev. Lett. 2013, 90, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Fernandez-Nieves, A.; Puertas, A. Fluids, Colloids and Soft Materials: An Introduction to Soft Matter Physics; J. Wiley and Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Likos, C.N. Effective interactions in soft condensed matter physics. Phys. Rep. 2001, 348, 267–439. [Google Scholar] [CrossRef]
- Pusey, P.N.; van Megen, W. Phase Behavior of Concentrated Suspensions of Nearly Hard Colloidal Spheres. Nature 1986, 320, 340–342. [Google Scholar] [CrossRef]
- Berthier, L. Theoretical perspective on the glass transition and amorphous materials. Rev. Mod. Phys. 2011, 83, 587. [Google Scholar] [CrossRef]
- Lu, P.J.; Zaccarelli, E.; Ciulla, F.; Schofield, A.B.; Sciortino, F.; Weitz, D.A. Gelation of particle with short range attraction. Nature 2008, 22, 499–503. [Google Scholar] [CrossRef]
- Royall, C.P.; Williams, S.R.; Ohtsuka, T.; Tanaka, H. Direct observation of a local structural mechanism for dynamic arrest. Nat. Mater. 2008, 7, 556–561. [Google Scholar] [CrossRef]
- Ruzicka, B.; Zaccarelli, E.; Zulian, L.; Angelini, R.; Sztucki, M.; Moussaïd, A.; Narayanan, T.; Sciortino, F. Observation of empty liquids and equilibrium gels in a colloidal clay. Nat. Mater. 2011, 10, 56–60. [Google Scholar] [CrossRef]
- Angelini, R.; Zaccarelli, E.; de Melo Marques, F.A.; Sztucki, M.; Fluerasu, A.; Ruocco, G.; Ruzicka, B. Glass–glass transition during aging of a colloidal clay. Nat. Commun. 2014, 5, 4049. [Google Scholar] [CrossRef] [Green Version]
- Pelton, R.H.; Chibante, P. Preparation of aqueous lattices with N-isopropylacrylamide. Colloids Surf. 1986, 20, 247–256. [Google Scholar] [CrossRef]
- Wu, X.; Pelton, R.H.; Hamielec, A.E.; Woods, D.R.; McPhee, W. The kinetics of poly(N-isopropylacrylamide) microgel latex formation. Colloid Polym. Sci. 1994, 272, 467–477. [Google Scholar] [CrossRef]
- Zhang, Y.; Furyk, S.; Bergbreiter, D.E.; Cremer, P.S. Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series. J. Am. Chem. Soc. 2005, 127, 14505–14510. [Google Scholar] [CrossRef]
- Sennato, S.; Chauveau, E.; Casciardi, S.; Bordi, F.; Truzzolillo, D. The double-faced electrostatic behavior of PNIPAm microgels. Polymers 2021, 13, 1153. [Google Scholar] [CrossRef]
- Lyon, L.A.; Fernandez-Nieves, A. The Polymer/Colloid Duality of Microgel Suspensions. Annu. Rev. Phys. Chem. 2012, 63, 25–43. [Google Scholar] [CrossRef]
- Annegarn, M.; Dirksen, M.; Hellweg, T. Importance of pH in Synthesis of pH-Responsive Cationic Nano-and Microgels. Polymers 2021, 13, 827. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.V. Colloidal microgels in drug delivery applications. Curr. Pharm. Des. 2006, 12, 4703–4712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeets, N.M.B.; Hoare, T. Designing responsive microgels for drug delivery applications. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3027–3043. [Google Scholar] [CrossRef]
- Fernández-Barbero, A.; Suárez, I.J.; Sierra-Martín, B.; Fernández-Nieves, A.; de Las Nieves, F.J.; Marquez, M.; Rubio-Retama, J.; López-Cabarcos, E. Gels and microgels for nanotechnological applications. Adv. Colloid Interface Sci. 2009, 147, 88–108. [Google Scholar] [CrossRef]
- Dirksen, M.; Kinder, T.A.; Brändel, T.; Hellweg, T. Temperature Controlled Loading and Release of the Anti-Inflammatory Drug Cannabidiol by Smart Microgels. Molecules 2021, 26, 3181. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.; Vinogradov, S. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [Green Version]
- Saunders, B.R.; Laajam, N.; Daly, E.; Teow, S.; Hu, X.; Stepto, R. Microgels: From responsive polymer colloids to biomaterials. Adv. Colloid Interface Sci. 2009, 147–148, 251–262. [Google Scholar] [CrossRef]
- Maya, S.; Sarmento, B.; Nair, A.; Rejinold, N.S.; Nair, S.V.; Jayakumar, R. Smart Stimuli Sensitive Nanogels in Cancer Drug Delivery and Imaging: A Review. Curr. Pharm. Des. 2013, 19, 7203–7218. [Google Scholar] [CrossRef] [PubMed]
- Meena, L.K.; Rather, H.; Kedaria, D.; Vasita, R. Polymeric microgels for bone tissue engineering applications—A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 381–397. [Google Scholar] [CrossRef]
- Newsom, J.P.; Payne, K.A.; Krebs, M.D. Microgels: Modular, Tunable Constructs for Tissue Regeneration. Acta Biomater. 2019, 88, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Okano, T. Stimuli-Responsive Hydrogels and Their Application to Functional Materials; Springer: New York, NY, USA, 2010; pp. 19–43. [Google Scholar]
- Rana, M.M.; la Hoz Siegler, H.D. Tuning the Properties of PNIPAm-Based Hydrogel Scaffolds for Cartilage Tissue Engineering. Polymers 2021, 13, 3154. [Google Scholar] [CrossRef]
- Argentiere, S.; Siciliano, P.A.; Blasi, L. How Microgels Can Improve the Impact of Organ-on-Chip and Microfluidic Devices for 3D Culture: Compartmentalization, Single Cell Encapsulation and Control on Cell Fate. Polymers 2021, 13, 3216. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nayak, S.; Lyon, L. Bioresponsive Hydrogel Microlenses. Am. Chem. Soc. 2005, 127, 9588–9592. [Google Scholar] [CrossRef] [PubMed]
- Serpe, M.; Kim, J.; Lyon, L. Colloidal Hydrogel Microlenses. Adv. Mater. 2004, 16, 184–187. [Google Scholar] [CrossRef]
- Nasimova, I.R.; Vyshivannaya, O.V.; Gallyamov, M.O.; Kozhunova, E.Y. Thermo- and pH-Sensitive Microgels Based on Interpenetrating Networks as Components for Creating Polymeric Materials. Polym. Sci. Ser. A 2019, 61, 773–779. [Google Scholar] [CrossRef]
- Nasimova, I.R.; Doroganov, V.Y.R.A.P.; Kharitonova, E.P.; Kozhunova, E.Y. Microstructured Macromaterials Based on IPN Microgels. Polymers 2021, 13, 1078. [Google Scholar] [CrossRef]
- Buratti, E.; Sanzari, I.; Dinelli, F.; Prodromakis, T.; Bertoldo, M. Formation and Stability of Smooth Thin Films with Soft Microgels Made of Poly(N-isopropylacrylamide) and Poly(acrylic acid). Polymers 2020, 12, 2638. [Google Scholar] [CrossRef]
- Cai, T.; Wang, G.; Thompson, S.; Marquez, M.; Hu, Z. Derivative Colloidal Spheres as Building Blocks. Macromolecules 2008, 41, 9508–9512. [Google Scholar] [CrossRef]
- Di Napoli, B.; Franco, S.; Severini, L.; Tumiati, M.; Buratti, E.; Titubante, M.; Nigro, V.; Gnan, N.; Micheli, L.; Ruzicka, B.; et al. Gellan Gum Microgels as Effective Agents for a Rapid Cleaning of Paper. ACS Appl. Polym. Mater. 2020, 2, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
- Scotti, A.; Brugnoni, M.; Lopez, C.G.; Bochenek, S.; Crassous, J.J.; Richtering, W. Flow properties reveal the particle-to-polymer transition of ultra-low crosslinked microgels. Soft Matter 2020, 16, 668–678. [Google Scholar] [CrossRef]
- Franco, S.; Buratti, E.; Ruzicka, B.; Nigro, V.; Zoratto, N.; Matricardi, P.; Zaccarelli, E.; Angelini, R. Volume fraction determination of microgel composed of interpenetrating polymer networks of PNIPAM and Polyacrylic acid. J. Phys. Condens. Matter 2021, 33, 174004. [Google Scholar] [CrossRef]
- Yunker, P.J.; Chen, K.; Gratale, M.D.; Lohr, M.A.; Still, T.; Yodh, A.G. Physics in ordered and disordered colloidal matter composed of poly(N-isopropyl acrylamide) microgel particles. Rep. Prog. Phys. 2014, 77, 056601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, Y.H.; Okano, T.; Kim, S.W. Temperature Dependence of Swelling of Crosslinked Poly(N,N′-alkyl substituted acrylamides) in Water. Polym. Phys. 1990, 98, 923–936. [Google Scholar] [CrossRef]
- Pelton, R. Poly(N-isopropylacrylamide)(PNIPAM) is never hydrophobic. J. Colloid Interface Sci. 2010, 348, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Nigro, V.; Angelini, R.; Bertoldo, M.; Ruzicka, B. Swelling of responsive-microgels: Experiments versus models. Colloids Surf. A 2017, A532, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Nigro, V.; Angelini, R.; Bertoldo, M.; Bruni, F.; Ricci, M.; Ruzicka, B. Local structure of temperature and pH-sensitive colloidal microgels. J. Chem. Phys. 2015, 143, 114904. [Google Scholar] [CrossRef]
- Kodre, K.V.; Attarde, S.R.; Yendhe, P.R.; Patil, R.Y.; Barge, V.U. Differential Scanning Calorimetry: A Review. Res. Rev. J. Pharm. Anal. 2014, 3, 11–22. [Google Scholar]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential Scanning Calorimetry Techniques: Applications in Biology and Nanoscience. J. Biomol. Tech. 2010, 21, 167–193. [Google Scholar] [PubMed]
- Ferrari, C.; Tombari, E.; Salvetti, G.; Johari, G. Composition dependence and the nature of endothermic freezing and exothermic melting. J. Chem. Phys. 2007, 126, 124506. [Google Scholar] [CrossRef]
- Sabín, J.; Prieto, G.; Sennato, S.; Ruso, J.M.; Angelini, R.; Bordi, F.; Sarmiento, F. Effect of Gd3+ on the colloidal stability of liposomes. Phys. Rev. E 2007, 74, 031913. [Google Scholar] [CrossRef] [Green Version]
- Angelini, R.; Ruocco, G.; Panfilis, S.D.; Sette, F. Phase diagram of a solution undergoing inverse melting. Phys. Rev. E 2008, 78, 020502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, L.J.; Li, B.; Wang, J.; Gu, Y.M. Application of DSC Technique in Study of Glass Ceramic. Adv. Mater. Res. 2010, 105–106, 743–745. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Martínez-Guerra, V.E.S.E.; Piñón-Balderrama, C.I.; Martínez, I.C.; Saavedra-Leos, M.Z. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries. Polymers 2019, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slobozeanu, A.E.; Bejan, S.E.; Tudor, I.A.; Mocioiu, A.M.; Motoc, A.M.; Romero-Sanchez, M.D.; Botan, M.; Catalin, C.G.; Madalina, C.L.; Piticescu, R.R.; et al. A review on differential scanning calorimetry as a tool for thermal assessment of nanostructured coatings. Manuf. Rev. 2021, 8, 1–15. [Google Scholar] [CrossRef]
- Kawasaki, H.; Sasaki, S.; Maeda, H. Effects of the Gel Size on the Volume Phase Transition of Poly(N-isopropylacrylamide) Gels: A Calorimetric Study. Langmuir 1998, 14, 773–776. [Google Scholar] [CrossRef]
- Grinberg, N.V.; Dubovik, A.S.; Grinberg, V.Y.; Kuznetsov, D.V.; Makhaeva, E.E.; Grosberg, A.Y.; Tanaka, T. Studies of the Thermal Volume Transition of Poly(N-isopropylacrylamide) Hydrogels by High-Sensitivity Differential Scanning Microcalorimetry. 1. Dynamic Effects. Macromolecules 1999, 32, 1471–1475. [Google Scholar] [CrossRef]
- Aangenendt, F.J.; Mattsson, J.; Ellenbroek, W.G.; Wyss, H.M. Mechanics from Calorimetry: Probing the elasticity Elasticity for Responsive Hydrogels. Phys. Rev. Appl. 2018, 8, 014003. [Google Scholar] [CrossRef] [Green Version]
- Woodward, N.C.; Chowdhry, B.Z.; Snowden, M.J.; Leharne, S.A.; Griffiths, P.C.; Winnington, A.L. Calorimetric Investigation of the Influence of Cross-Linker Concentration on the Volume Phase Transition of Poly(N-isopropylacrylamide) Colloidal Microgels. Langmuir 2003, 19, 3202–3211. [Google Scholar] [CrossRef]
- Hoare, T.; Pelton, R. Calorimetric Analysis of Thermal Phase Transitions in Functionalized Microgels. J. Phys. Chem. 2007, 111, 1334–1342. [Google Scholar] [CrossRef]
- Guo, Y.X.; Liu, Y.D.; Liu, R.; Tian, Y.; Chen, K.; Wang, L.M. Direct evidence of entropy driven fluid-like–glass-like transition in microgel suspensions. Appl. Phys. Lett. 2017, 110, 071902. [Google Scholar] [CrossRef]
- Zanatta, M.; Orecchini, A.; Tavagnacco, L.; Buratti, E.; Chiessi, E.; Natali, F.; Bertoldo, M.; Zaccarelli, E. Atomic scale investigation of the volume phase transition in concentrated PNIPAM microgels. J. Chem. Phys. 2020, 152, 204904. [Google Scholar] [CrossRef]
- Berndt, I.; Popescu, C.; Wortmann, F.J.; Richtering, W. Mechanics versus Thermodynamics: Swelling in Multiple-Temperature Sensitive Core–Shell Microgels. Angew. Chem. 2006, 45, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Buratti, E.; Tavagnacco, L.; Zanatta, M.; Chiessi, E.; Franco, S.; Ruzicka, B.; Angelini, R.; Orecchini, A.; Bertoldo, M.; Zaccarelli, E. The role of polymer structure on water confinement in poly(N-isopropylacrylamide) dispersions. J. Mol. Liq. 2021; unpublishedwork. [Google Scholar]
- Snowden, M.J.; Chowdhry, B.Z.; Vincent, B.; Morris, G.E. Colloidal copolymer microgels of N-isopropylacrylamide and acrylic acid: pH, ionic strength and temperature effects. J. Chem. Soc. Faraday Trans. 1996, 92, 24. [Google Scholar] [CrossRef]
- Oropeza, M.V.C.; Souza, E.F.; Giudici, R. Study of the Porosity of the Microgel of pAA and Co-Polymer Microgel of p(AA-co-NIPAM) Through the Thermoporometry Technique. Macromol. Symp. 2016, 367, 24–29. [Google Scholar] [CrossRef]
- Grinberg, V.Y.; Burova, T.V.; Grinberg, N.V.; Buyanovskaya, A.G.; Khokhlov, A.R.; Kozhunova, E.Y.; Vyshivannaya, O.V.; Nasimova, I.R. Functionalized thermoresponsive microgels based on N-isopropylacrylamide: Energetics and mechanism of phase transitions. Eur. Polym. J. 2020, 133, 109722. [Google Scholar] [CrossRef]
- Nigro, V.; Angelini, R.; Bertoldo, M.; Buratti, E.; Franco, S.; Ruzicka, B. Chemical-Physical Behaviour of Microgels Made of Interpenetrating Polymer Networks of PNIPAM and Poly(acrylic Acid). Polymers 2021, 13, 1353. [Google Scholar] [CrossRef]
- Xia, X.; Hu, Z. Synthesis and Light Scattering Study of Microgels with Interpenetrating Polymer Networks. Langmuir 2004, 20, 2094–2098. [Google Scholar] [CrossRef]
- Hu, Z.; Xia, X. Hydrogel nanoparticle dispersions with inverse thermoreversible gelation. Adv. Mater. 2004, 16, 305–309. [Google Scholar] [CrossRef]
- Micali, N.; Bertoldo, M.; Buratti, E.; Nigro, V.; Angelini, R.; Villari, V. Interpenetrating Polymer Network Microgels in Water: Effect of Composition on the Structural Properties and Electrosteric Interactions. ChemPhysChem 2018, 19, 2894–2901. [Google Scholar] [CrossRef]
- Höhne, G.; McNaughton, J.; Hemminger, W.; Flammersheim, H.J.; Flammersheim, H.J. Differential Scanning Calorimetry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Sarge, S.M.; Höhne, G.W.; Hemminger, W. Calorimetry: Fundamentals, Instrumentation and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Kohlrausch, R. Thermoresponsive poly-(N-isopropylmethacrylamide) microgels: Tailoring particle size by interfacial tension control. Pogg. Ann. Phys. Chem. 1854, 91, 179–214. [Google Scholar] [CrossRef] [Green Version]
- Williams, G.; Watts, D.C. Non-symmetrical dielectric relaxation behavior arising from a simple empirical decay function. J. Chem. Soc. Faraday Trans. 1970, 66, 80–85. [Google Scholar] [CrossRef]
- Nigro, V.; Angelini, R.; Rosi, B.; Bertoldo, M.; Buratti, E.; Casciardi, S.; Sennato, S.; Ruzicka, B. Study of network composition in interpenetrating polymer networks of poly(N isopropylacrylamide) microgels: The role of poly(acrylic acid). J. Colloid Interface Sci. 2019, 545, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Höhne, G.W.H.; Hemminger, W.F.; Flammersheim, H.J. Differential Scanning Calorimetry; Springer: New York, NY, USA, 2003. [Google Scholar]
- Franco, S.; Buratti, E.; Nigro, V.; Zaccarelli, E.; Ruzicka, B.; Angelini, R. Glass and Jamming Rheology in Soft Particles Made of PNIPAM and Polyacrylic Acid. Int. J. Mol. Sci. 2021, 22, 4032. [Google Scholar] [CrossRef] [PubMed]
- Nigro, V.; Ruzicka, B.; Ruta, B.; Zontone, F.; Bertoldo, M.; Buratti, E.; Angelini, R. Relaxation Dynamics, Softness, and Fragility of Microgels with Interpenetrated Polymer Networks. Macromolecules 2020, 53, 1596–1603. [Google Scholar] [CrossRef]
- Stieger, M.; Richtering, W.; Pedersen, J.S.; Lindner, P. Small-angle neutron scattering study of structural changes in temperature sensitive microgel colloids. J. Chem. Phys. 2004, 120, 6197–6206. [Google Scholar] [CrossRef]
- Romeo, G.; Ciamarra, M.P. Elasticity of compressed microgel suspensions. Soft Matter 2013, 9, 5401–5406. [Google Scholar] [CrossRef] [Green Version]
- Swift, T.; Swanson, L.; Geoghegan, M.; Rimmer, S. The pH-responsive behaviour of poly(acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter 2016, 12, 2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anghel, D.F.; Alderson, V.; Winnik, F.M.; Mizusaki, M.; Morishima, Y. Fluorescent dyes as model ‘hydrophobic modifiers’ of polyelectrolytes: A study of poly(acrylic acid)s labelled with pyrenyl and naphthyl groups. Polymer 1998, 39, 3035–3044. [Google Scholar] [CrossRef]
- Arnold, R. The titration of polymeric acids. J. Colloid Sci. 1957, 12, 549–556. [Google Scholar] [CrossRef]
- Hanykovà, L.; Krakovskỳ, I.; Šestàkovà, E.; Št’astnà, J.; Labuta, J. Poly(N,N′-Diethylacrylamide)-Based Thermoresponsive Hydrogels with Double Network Structure. Polymers 2020, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- Katchalsky, A.; Eisenberg, H. Molecular weight of polyacrylic and polymethacrylic acid. J. Polym. Sci. 1951, 6, 145–154. [Google Scholar] [CrossRef]










| Reference | Investigated System | Highlighted Topic | Investigation Techniques |
|---|---|---|---|
| Sennato et al. [16] | PNIPAM microgels | electrostatic behaviour | DLS, TEM, AFM, electrophoresis, viscosimetry |
| Annegarn et al. [18] | P(NIPAM-co-APMH) microgels | electrostatic behaviour | DLS, AFM, H-NMR |
| Nasimova et al. [34] | Macromaterials of PNIPAM-PAAc IPN microgels | macromaterial properties | FTIR, computer simulations |
| Buratti et al. [35] | Films of PNIPAM-PAAc IPN microgels | layer characterization | DLS, H-NMR, AFM contact angle |
| Nigro et al. [65] | PNIPAM-PAAc IPN microgels | physical behaviour | DLS, SANS, Raman, rheology, electrophoresis |
| 2.7 | 2.19 | 94.5 | 2.8 | ||
| 4.5 | 2.13 | 91.5 | 4.0 | ||
| 10.6 | 1.79 | 88.3 | 1.1 | ||
| 15.4 | 1.36 | 79.2 | 5.4 | ||
| 24.6 | 1.22 | 67.7 | 7.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, S.; Buratti, E.; Nigro, V.; Bertoldo, M.; Ruzicka, B.; Angelini, R. Thermal Behaviour of Microgels Composed of Interpenetrating Polymer Networks of Poly(N-isopropylacrylamide) and Poly(acrylic acid): A Calorimetric Study. Polymers 2022, 14, 115. https://doi.org/10.3390/polym14010115
Franco S, Buratti E, Nigro V, Bertoldo M, Ruzicka B, Angelini R. Thermal Behaviour of Microgels Composed of Interpenetrating Polymer Networks of Poly(N-isopropylacrylamide) and Poly(acrylic acid): A Calorimetric Study. Polymers. 2022; 14(1):115. https://doi.org/10.3390/polym14010115
Chicago/Turabian StyleFranco, Silvia, Elena Buratti, Valentina Nigro, Monica Bertoldo, Barbara Ruzicka, and Roberta Angelini. 2022. "Thermal Behaviour of Microgels Composed of Interpenetrating Polymer Networks of Poly(N-isopropylacrylamide) and Poly(acrylic acid): A Calorimetric Study" Polymers 14, no. 1: 115. https://doi.org/10.3390/polym14010115
APA StyleFranco, S., Buratti, E., Nigro, V., Bertoldo, M., Ruzicka, B., & Angelini, R. (2022). Thermal Behaviour of Microgels Composed of Interpenetrating Polymer Networks of Poly(N-isopropylacrylamide) and Poly(acrylic acid): A Calorimetric Study. Polymers, 14(1), 115. https://doi.org/10.3390/polym14010115