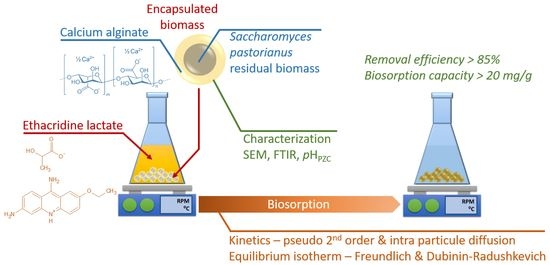
Encapsulation of Saccharomyces pastorianus Residual Biomass in Calcium Alginate Matrix with Insights in Ethacridine Lactate Biosorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Analytical Procedure
2.2. Biosorbent Synthesis and Characterization
2.3. Influence of Biosorption Operational Parameters (pH, Adsorbent Amount, Initial Pollutant Concentration, Working Temperature)
2.4. Kinetics and Equilibrium Isotherms
3. Results and Discussion
3.1. Biosorbent Preparation
3.2. Biosorbent Characterization (SEM, FTIR, and Point of Zero Charge)
3.3. Influence of Working Parameters (pH, Biosorbent Dosage, EL Initial Concentration and Temperature) on the Biosorption Process
3.3.1. Influence of pH
3.3.2. Influence of Biosorbent Dosage
3.3.3. Influence of EL Initial Concentration
3.3.4. Influence of Temperature
3.4. Biosorption Kinetics
3.5. Equilibrium Isotherms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adewuyi, A. Chemically modified biosorbents and their role in the removal of emerging pharmaceutical waste in the water system. Water 2020, 12, 1551. [Google Scholar] [CrossRef]
- Talman, R.Y.C.; Salİhİ, E.C.; Gokturk, S.; Bastug, A.S. Removal of ethacridine lactate from aqueous solutions onto bentonite and activated carbon. Fresenius Environ. Bull. 2015, 24, 3603–3608. [Google Scholar]
- Mojiri, A.; Vakili, M.; Farraji, H.; Aziz, S.Q. Combined ozone oxidation process and adsorption methods for the removal of acetaminophen and amoxicillin from aqueous solution; kinetic and optimisation. Environ. Technol. Innov. 2019, 15, 100404. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ratnaweera, H.; Rezania, S.; Nazari, V.M. Pharmaceuticals and personal care products in aquatic environments and their removal by algae-based systems. Chemosphere 2022, 288, 132580. [Google Scholar] [CrossRef]
- Lin, J.; Su, T.; Chen, J.; Xue, T.; Yang, S.; Guo, P.; Lin, H.; Wang, H.; Hong, Y.; Su, Y.; et al. Efficient adsorption removal of anionic dyes by an imidazolium-based mesoporous poly(ionic liquid) including the continuous column adsorption-desorption process. Chemosphere 2021, 272, 129640. [Google Scholar] [CrossRef]
- Kais, H.; Yeddou Mezenner, N.; Trari, M. Biosorption of rifampicin from wastewater using cocoa shells product. Sep. Sci. Technol. 2020, 55, 1984–1993. [Google Scholar] [CrossRef]
- Yaqubi, O.; Tai, M.; Mitra, D.; Gerente, C.; Neoh, K.; Wang, C.-H.; Andres, Y. Adsorptive removal of tetracycline and amoxicillin from aqueous solution by leached carbon black waste and chitosan-carbon composite beads. J. Environ. Chem. Eng. 2021, 9, 104988. [Google Scholar] [CrossRef]
- Mittal, A.; Mittal, J.; Kurup, L. Adsorption isotherms, kinetics and column operations for the removal of hazardous dye, Tartrazine from aqueous solutions using waste materials—Bottom ash and de-oiled soya, as adsorbents. J. Hazard. Mater. 2006, 136, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Felis, E.; Kalka, J.; Sochacki, A.; Kowalska, K.; Bajkacz, S.; Harnisz, M.; Korzeniewska, E. Antimicrobial pharmaceuticals in the aquatic environment—Occurrence and environmental implications. Eur. J. Pharmacol. 2020, 866, 172813. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Rusu, L.; Suceveanu, M.; Șuteu, D.; Favier, L.; Harja, M. Assessment of groundwater and surface water contamination by landfill leachate: A case study in Neamț County, Romania. Environ. Eng. Manag. J. 2017, 16, 633–641. [Google Scholar] [CrossRef]
- Kadmi, Y.; Favier, L.; Soutrel, I.; Lemasle, M.; Wolbert, D. Ultratrace-level determination of N-Nitrosodimethylamine, N-Nitrosodiethylamine, and N-Nitrosomorpholine in waters by solid-phase extraction followed by liquid chromatography-tandem mass spectrometry. Open Chem. 2014, 12, 928–936. [Google Scholar] [CrossRef]
- Vrinceanu, N.; Hlihor, R.M.; Simion, A.I.; Rusu, L.; Fekete-Kertész, I.; Barka, N.; Favier, L. New evidence of the enhanced elimination of a persistent drug used as a lipid absorption inhibitor by advanced oxidation with UV-A and nanosized catalysts. Catalysts 2019, 9, 761. [Google Scholar] [CrossRef] [Green Version]
- Favier, L.; Simion, A.I.; Rusu, L.; Pacala, M.L.; Grigoraş, C.-G.; Bouzaza, A. Removal of an organic refractory compound by photocatalysis in batch reactor—A kinetic study. Environ. Eng. Manag. J. 2015, 14, 1327–1338. [Google Scholar] [CrossRef]
- Popa Ungureanu, C.; Favier, L.; Bahrim, G. Screening of soil bacteria as potential agents for drugs biodegradation: A case study with clofibric acid. J. Chem. Technol. Biotechnol. 2016, 91, 1646–1653. [Google Scholar] [CrossRef]
- Favier, L.; Rusu, L.; Simion, A.I.; Hlihor, R.M.; Păcală, M.L.; Augustyniak, A. Efficient degradation of clofibric acid by heterogeneous photocatalytic oxidation process. Environ. Eng. Manag. J. 2019, 18, 1683–1692. [Google Scholar] [CrossRef]
- De Rossi, A.; Rigueto, C.V.T.; Dettmer, A.; Colla, L.M.; Piccin, J.S. Synthesis, characterization, and application of Saccharomyces cerevisiae/alginate composites beads for adsorption of heavy metals. J. Environ. Chem. Eng. 2020, 8, 104009. [Google Scholar] [CrossRef]
- Kordialik-Bogacka, E. Saccharomyces pastorianus immobilized on brewer’s spent grain in continuous system for lead ion biosorption. Int. Biodeterior. Biodegrad. 2014, 96, 191–197. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Asgari, E.; Shokoohi, R.; Dargahi, A.; Arabkoushsar, A. Removing amoxicillin antibiotic from aquoues solutions by Saccharomyces cerevisiae bioadsorbent: Kinetic, thermodynamic and isotherm studies. Desalination Water Treat. 2019, 152, 306–315. [Google Scholar] [CrossRef]
- Moreno Rivas, S.C.; Armenta Corral, R.I.; Frasquillo Félix, M.d.C.; Islas Rubio, A.R.; Vázquez Moreno, L.; Ramos-Clamont Montfort, G. Removal of cadmium from aqueous solutions by Saccharomyces cerevisiae–alginate system. Materials 2019, 12, 4128. [Google Scholar] [CrossRef] [Green Version]
- Mojiri, A.; Andasht Kazeroon, R.; Gholami, A. Cross-linked magnetic chitosan/activated biochar for removal of emerging micropollutants from water: Optimization by the Artificial Neural Network. Water 2019, 11, 551. [Google Scholar] [CrossRef] [Green Version]
- Karimi-Maleh, H.; Ayati, A.; Davoodi, R.; Tanhaei, B.; Karimi, F.; Malekmohammadi, S.; Orooji, Y.; Fu, L.; Sillanpää, M. Recent advances in using of chitosan-based adsorbents for removal of pharmaceutical contaminants: A review. J. Clean. Prod. 2021, 291, 125880. [Google Scholar] [CrossRef]
- Dilarri, G.; Corso, C.R. Saccharomyces cerevisiae immobilized onto cross-linked chitosan beads: Application of a novel material for the removal of dye toxicity. Environ. Technol. 2018, 39, 1851–1867. [Google Scholar] [CrossRef] [PubMed]
- Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.M.; Șuteu, D.; Harja, M. Application of Saccharomyces cerevisiae/calcium alginate composite beads for cephalexin antibiotic biosorption from aqueous solutions. Materials 2021, 14, 4728. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Arnold, W.A.; Novak, P.J. Encapsulation technology to improve biological resource recovery: Recent advancements and research opportunities. Environ. Sci. Water Res. Technol. 2021, 7, 16–23. [Google Scholar] [CrossRef]
- Umar Mustapha, M.; Halimoon, N. Microorganisms and biosorption of heavy metals in the environment: A review paper. J. Microb. Biochem. Technol. 2015, 7, 253–256. [Google Scholar] [CrossRef]
- Trojanowska, A.; Nogalska, A.; Valls, R.G.; Giamberini, M.; Tylkowski, B. Technological solutions for encapsulation. Phys. Sci. Rev. 2017, 2, 20170020. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Racioppo, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Microencapsulation of Saccharomyces cerevisiae into alginate beads: A focus on functional properties of released cells. Foods 2020, 9, 1051. [Google Scholar] [CrossRef] [PubMed]
- Rusu, L.; Grigoraș, C.-G.; Suceveanu, E.M.; Simion, A.-I.; Dediu Botezatu, A.V.; Istrate, B.; Doroftei, I. Eco-friendly biosorbents based on microbial biomass and natural polymers: Synthesis, characterization and application for the removal of drugs and dyes from aqueous solutions. Materials 2021, 14, 4810. [Google Scholar] [CrossRef]
- Massoud, R.; Khosravi-Darani, K.; Sharifan, A.; Asadi, G.H. Lead bioremoval from milk by Saccharomyces cerevisiae. Biocatal. Agric. Biotechnol. 2019, 22, 101437. [Google Scholar] [CrossRef]
- Conway, J. Beer Production Worldwide from 1998 to 2020 (in Billion Hectoliters). 2021. Available online: https://www.statista.com/statistics/270275/worldwide-beer-production/ (accessed on 4 November 2021).
- Aronson, J.K. (Ed.) Ethacridine. In Meyler’s Side Effects of Drugs, 16th ed.; Elsevier: Oxford, UK, 2016; p. 171. [Google Scholar]
- Iliescu, T.; Baia, M.; Maniu, D. Raman and surface enhanced Raman spectroscopy on molecules of pharmaceutical and biological interest. Rom. Rep. Phys. 2008, 60, 829–855. [Google Scholar]
- Talman, R.Y.; Gokturk, S.; Çalışkan, E.; Bastug, A. Adsorption kinetics of rivanol on activated carbon from aqueous solutions. In Proceedings of the 6th European Meeting on Chemical Industry and Environment, Mechelen, Belgium, 17–19 May 2010. [Google Scholar]
- Dadwal, V.; Joshi, R.; Gupta, M. Formulation, characterization and in vitro digestion of polysaccharide reinforced Ca-alginate microbeads encapsulating Citrus medica L. phenolics. LWT 2021, 152, 112290. [Google Scholar] [CrossRef]
- Das, T.K.; Scott, Q.; Bezbaruah, A.N. Montmorillonite-iron crosslinked alginate beads for aqueous phosphate removal. Chemosphere 2021, 281, 130837. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Wang, R.; Rao, P.; Yan, L.; Zhang, W.; Wang, J.; Chai, F. The fabrication of calcium alginate beads as a green sorbent for selective recovery of Cu(II) from metal mixtures. Crystals 2019, 9, 255. [Google Scholar] [CrossRef] [Green Version]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef] [Green Version]
- Sathisaran, I.; Balasubramanian, M. Physical characterization of chitosan/gelatin-alginate composite beads for controlled release of urea. Heliyon 2020, 6, e05495. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, J.; Xue, R.; Yang, Y. Spectroscopic characterization of Au3+ biosorption by waste biomass of Saccharomyces cerevisiae. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 761–765. [Google Scholar] [CrossRef]
- Kowalczuk, D.; Pitucha, M. Application of FTIR method for the assessment of immobilization of active substances in the matrix of biomedical materials. Materials 2019, 12, 2972. [Google Scholar] [CrossRef] [Green Version]
- Marjanović, B.; Juranić, I.; Ćirić-Marjanović, G.; Mojović, M.; Pašti, I.; Janošević, A.; Trchová, M.; Holler, P.; Horský, J. Chemical oxidative polymerization of ethacridine. React. Funct. Polym. 2012, 72, 25–35. [Google Scholar] [CrossRef]
- White, J. Variation in water content of yeast cells caused by varying temperatures of growth and by other cultural conditions. J. Inst. Brew. 1952, 58, 47–50. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Okada, S.; Nakahara, H.; Isaka, H. Adsorption of drugs on microcrystalline cellulose suspended in aqueous solutions. Chem. Pharm. Bull. 1987, 35, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Adel Niaei, H.; Rostamizadeh, M. Adsorption of metformin from an aqueous solution by Fe-ZSM-5 nano-adsorbent: Isotherm, kinetic and thermodynamic studies. J. Chem. Thermodyn. 2020, 142, 106003. [Google Scholar] [CrossRef]
- Asgari, E.; Sheikhmohammadi, A.; Yeganeh, J. Application of the Fe3O4-chitosan nano-adsorbent for the adsorption of metronidazole from wastewater: Optimization, kinetic, thermodynamic and equilibrium studies. Int. J. Biol. Macromol. 2020, 164, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, O.; Fouad, Y.O.; Amin, N.K.; Mandor, H. Kinetic and thermodynamic aspects of cadmium adsorption onto raw and activated guava (Psidium guajava) leaves. Environ. Prog. Sustain. Energy 2015, 34, 351–358. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.S.; Muñoz-Peña, M.J.; Domínguez-Vargas, J.R.; González, T.; Cuerda-Correa, E.M. Kinetic and equilibrium adsorption parameters estimation based on a heterogeneous intraparticle diffusion model. Surf. Interfaces 2021, 22, 100791. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Susan, M.A.B.H.; Carabineiro, S.A.C. Adsorption of cationic dyes, drugs and metal from aqueous solutions using a polymer composite of magnetic/β-cyclodextrin/activated charcoal/Na alginate: Isotherm, kinetics and regeneration studies. J. Hazard. Mater. 2021, 409, 124840. [Google Scholar] [CrossRef]
- Costa, R.L.T.; do Nascimento, R.A.; de Araújo, R.C.S.; Vieira, M.G.A.; da Silva, M.G.C.; de Carvalho, S.M.L.; de Faria, L.J.G. Removal of non-steroidal anti-inflammatory drugs (NSAIDs) from water with activated carbons synthetized from waste murumuru (Astrocaryum murumuru Mart.): Characterization and adsorption studies. J. Mol. Liq. 2021, 343, 116980. [Google Scholar] [CrossRef]
- Suriyanon, N.; Punyapalakul, P.; Ngamcharussrivichai, C. Mechanistic study of diclofenac and carbamazepine adsorption on functionalized silica-based porous materials. Chem. Eng. J. 2013, 214, 208–218. [Google Scholar] [CrossRef]
- Turk Sekulic, M.; Boskovic, N.; Slavkovic, A.; Garunovic, J.; Kolakovic, S.; Pap, S. Surface functionalised adsorbent for emerging pharmaceutical removal: Adsorption performance and mechanisms. Process Saf. Environ. Prot. 2019, 125, 50–63. [Google Scholar] [CrossRef]
- Vieira, J.C.; Soares, L.C.; Froes-Silva, R.E.S. Comparing chemometric and Langmuir isotherm for determination of maximum capacity adsorption of arsenic by a biosorbent. Microchem. J. 2018, 137, 324–328. [Google Scholar] [CrossRef]
- Chung, H.-K.; Kim, W.-H.; Park, J.; Cho, J.; Jeong, T.-Y.; Park, P.-K. Application of Langmuir and Freundlich isotherms to predict adsorbate removal efficiency or required amount of adsorbent. J. Ind. Eng. Chem. 2015, 28, 241–246. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Fadzail, F.; Hasan, M.; Mokhtar, Z.; Ibrahim, N. Removal of naproxen using low-cost Dillenia Indica peels as an activated carbon. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Youssef, A.M.; EL-Khouly, S.M.; El-Nabarawy, T. Removal of Pb(II) and Cd(II) from aqueous solution using oxidized activated carbons developed from pecan shells. Carbon Lett. 2008, 9, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Gholamiyan, S.; Hamzehloo, M.; Farrokhnia, A. RSM optimized adsorptive removal of erythromycin using magnetic activated carbon: Adsorption isotherm, kinetic modeling and thermodynamic studies. Sustain. Chem. Pharm. 2020, 17, 100309. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, J.-H. Isotherm, kinetic and thermodynamic studies on the adsorption of paclitaxel onto Sylopute. J. Chem. Thermodyn. 2019, 130, 104–113. [Google Scholar] [CrossRef]








| Kinetic Model | EL Initial Concentration, Mg/L | Kinetic Parameters | Correlation Coefficient, R2 | |||
|---|---|---|---|---|---|---|
| Qe | K2 | Ki | C | |||
| Pseudo-second-order | 20 | 5.6932 | 0.0031 | - | - | 0.9917 |
| 30 | 9.2236 | 0.0062 | - | - | 0.9927 | |
| 40 | 13.0694 | 0.0086 | - | - | 0.9906 | |
| 50 | 17.1651 | 0.0144 | - | - | 0.9944 | |
| 60 | 21.3909 | 0.0194 | - | - | 0.9951 | |
| Intraparticle diffusion | 20 | - | - | 0.4321 | 0.0587 | 0.9755 |
| 30 | - | - | 0.6879 | 0.3664 | 0.9696 | |
| 40 | - | - | 0.9889 | 0.4344 | 0.9703 | |
| 50 | - | - | 1.2558 | 1.1923 | 0.9651 | |
| 60 | - | - | 1.5669 | 1.6801 | 0.9618 | |
| Parameter | Freundlich Model | Dubinin–Radushkevich Model |
|---|---|---|
| n | 0.5232 | - |
| KF (mg/g) | 2.8394 | - |
| qm (mg/g) | - | 28.8765 |
| β (mol2/kJ2) | - | 0.000023 |
| E (kJ/mol) | - | 147.4420 |
| R2 | 0.9942 | 0.9369 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.-M.; Blaga, A.-C.; Harja, M. Encapsulation of Saccharomyces pastorianus Residual Biomass in Calcium Alginate Matrix with Insights in Ethacridine Lactate Biosorption. Polymers 2022, 14, 170. https://doi.org/10.3390/polym14010170
Rusu L, Grigoraș C-G, Simion A-I, Suceveanu E-M, Blaga A-C, Harja M. Encapsulation of Saccharomyces pastorianus Residual Biomass in Calcium Alginate Matrix with Insights in Ethacridine Lactate Biosorption. Polymers. 2022; 14(1):170. https://doi.org/10.3390/polym14010170
Chicago/Turabian StyleRusu, Lăcrămioara, Cristina-Gabriela Grigoraș, Andrei-Ionuț Simion, Elena-Mirela Suceveanu, Alexandra-Cristina Blaga, and Maria Harja. 2022. "Encapsulation of Saccharomyces pastorianus Residual Biomass in Calcium Alginate Matrix with Insights in Ethacridine Lactate Biosorption" Polymers 14, no. 1: 170. https://doi.org/10.3390/polym14010170
APA StyleRusu, L., Grigoraș, C. -G., Simion, A. -I., Suceveanu, E. -M., Blaga, A. -C., & Harja, M. (2022). Encapsulation of Saccharomyces pastorianus Residual Biomass in Calcium Alginate Matrix with Insights in Ethacridine Lactate Biosorption. Polymers, 14(1), 170. https://doi.org/10.3390/polym14010170