A Review on Nanocellulose and Superhydrophobic Features for Advanced Water Treatment
Abstract
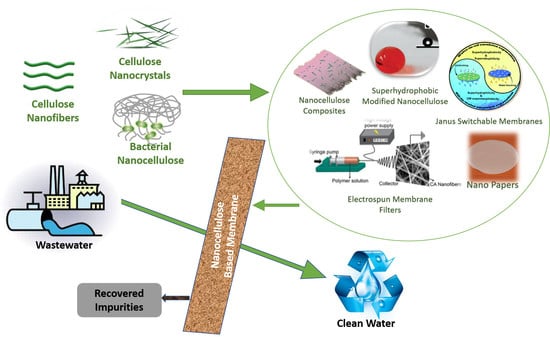
:1. Introduction
2. Structure and Properties of Nanocellulose
2.1. Cellulose Nanofibers (CNFs) Structure and Properties
2.2. Cellulose Nanocrystals (CNCs) Traits and Structure
2.3. Bacterial Nanocelluloses (BNCs) Traits and Structure
3. Nanocellulose Sources
4. Nanocellulose Preparation
4.1. Acid Hydrolyzation
4.2. Oxidative Process
4.3. Physical Process
4.4. Ionic Liquid Process
4.5. Biological Process
4.6. Electrospinning Method
5. Cellulose-Based Materials in Industrial-Scale Water Treatment
6. Superhydrophobic Nanocellulose-Based Wastewater Treatment Materials
6.1. Theory of Pollutant Separation across Hydrophobic Surfaces or Interfaces
6.2. Models in Exploratory Research
7. The Applications of Hydrophobic/Superhydrophobic Structures
7.1. Removal of Heavy Metals
7.1.1. Heavy Metals in the Environment
7.1.2. Nanocellulose-Based Materials for Adsorption
| Adsorbent Type | Targeted Heavy Metal | Production Method | Optimum Condition | Maximum Adsorption mg/g | Reference |
|---|---|---|---|---|---|
| TEMPO-oxidized CNF with PEI | Cu (II) | TEMPO oxidized cellulose nanofibers (TOCNF) grafted with Polyethylenimine (PEI) | Room Temperature/pH 5–7 | 52.32 | [114] |
| TEMPO-oxidized CNF | Cu (II) | TEMPO oxidized cellulose nanofibers (TOCNF) from beech pulp fibers were prepared by using 10 mmol/g of NaClO. | Room Temperature/pH 7 | 135 | [124] |
| Carboxylated cellulose nanocrystal-sodium alginate (CCN-Alg) hydrogel beads | Pb (II) | CCN-Alg adsorbent material prepared from sodium alginate in CaCl2 solution. | Room Temperature/pH 5.2/Contact time 180 min | 338.98 | [125] |
| Shape memory aerogels from nanofibrillated cellulose(NFC) and polyethyleneimine(PEI) | Cu(II) and Pb(II) | NPAs of NFC and PEI manufactured in an easy and green method approach via electrostatic blend. | Room Temperature/pH 2~5 | 175.44 & 357.14, respectively | [126] |
| Nanocrystalline cellulose (NCC) | Cr(III) & Cr(VI) | reinforcement using succination and amination | Room Temperature/pH 2.5~6.5 | Cr(III) (94.84%) and Cr(VI) (98.33%) | [127] |
| Thiourea-functionalized magnetic ZnO/nanocellulose composite(TZFNC) | Pb (II) | TZFNC composite was manufactured by using a simple chemical co-precipitation technique. | Room Temperature/pH 6.5/Contact time 14.5 min | 554.4 | [128] |
| Surface functionalization of cellulose nano fibers by using methionine (Meth-CNF) | Hg (II) | CNFs extracted from rice straw were functionalized using l-methionine | Room Temperature/pH 7.8 | 131.86 | [129] |
| CNC modified with NaNO2/NaHCO3 | Ni (II) | sawdust-derived cellulose nanocrystals (CNC) coagulant | Room Temperature/pH 7.10 | 956.6 | [130] |
| CNC | Pb (II) | Cellulose nanocrystal (CNC) from cassava peel by acid hydrolysis | Room Temperature/pH 6/Contact time 30 min | 6.4 | [131] |
| NC-PEI/GA | As (v) | Nanocellulose cross-linking polyethyleneimine and glutaraldehyde. | Room Temperature/pH 3 | 255.19 | [132] |
| CNC | Pb (II) | developed from agricultural waste Oryza sativa husk synthesized by chemo-mechanical | Room Temperature/pH 8/Contact time 70 min | 3.783 | [133] |
| CNC/iron oxide nanorod composites | As (III) & As (V) | Acid catalyzed hydrolysis of microcrystalline cellulose | Room temperature/pH 5–9/Contact time 2 h | 13.87 | [134] |
| Poly(acryloylhydrazide)-grafted CNC Fe–Cu alloy coated CNC | Cr(VI) & Pb(II) | Atom transfer radical polymerization Oxidation-reduction method | Room temperature/pH 3 | 45.7 & 81.94 | [135,136] |
7.2. Removal of Dyes
7.3. Filtration and Separation Media for Wastewater Treatment
- A series of pre-treatment processes;
- Considerable energy consumption owing to high pressure;
- Serious membrane fouling.
7.3.1. Superhydrophobic Modified Nanocellulose for Oil/Water Separation
Janus Switchable Membranes
7.3.2. Electrospun Membrane Filters
7.3.3. Nanopapers as Membrane Filters
7.3.4. Membrane Bioreactors
8. Sustainability, Challenges, and Limitations
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, D.; Cai, J.; Xu, W.; Dong, Q.; Li, Y.; Liu, G.; Wang, Z. Synthesis, characterization and adsorption property of cellulose nanofiber-based hydrogels. J. For. Eng. 2019, 4, 92–98. [Google Scholar]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose-based materials for water purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Jamaly, S.; Giwa, A.; Hasan, S.W. Recent improvements in oily wastewater treatment: Progress, challenges, and future opportunities. J. Environ. Sci. 2015, 37, 15–30. [Google Scholar] [CrossRef] [PubMed]
- WHO/UNICEF. 2021. Available online: https://www.unicef.org/publications/index_19033.html (accessed on 12 August 2021).
- SupWHO/UNICEF Joint Water Supply; Sanitation Monitoring Programme. Progress on Sanitation and Drinking Water: 2015 Update and MDG Assessment; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. Lack of Sanitation for 2.4 Billion People is Undermining Health Improvements; WHO: New York, NY, USA, 2015. [Google Scholar]
- WHO/UNICEF. 2021. Available online: https://www.unicef.org/publications/index_82419.html (accessed on 12 August 2021).
- Fenwick, A. Waterborne infectious diseases—Could they be consigned to history? Science 2006, 313, 1077–1081. [Google Scholar] [CrossRef] [Green Version]
- Peng, B.; Yao, Z.; Wang, X.; Crombeen, M.; Sweeney, D.G.; Tam, K.C. Cellulose-based materials in wastewater treatment of petroleum industry. Green Energy Environ. 2020, 5, 37–49. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Kuusipalo, J. Superhydrophobic coatings on cellulose-based materials: Fabrication, properties, and applications. Adv. Mater. Interfaces 2014, 1, 1300026. [Google Scholar] [CrossRef]
- Panchal, P.; Ogunsona, E.; Mekonnen, T. Trends in advanced functional material applications of nanocellulose. Processes 2019, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Tshikovhi, A.; Mishra, S.B.; Mishra, A.K. Nanocellulose-based composites for the removal of contaminants from wastewater. Int. J. Biol. Macromol. 2020, 152, 616–632. [Google Scholar] [CrossRef]
- Heidi Lynn, R.; Priscilla Gl, B.; Emmanuel, I. Metal nanoparticle modified polysulfone membranes for use in wastewater treatment: A critical review. J. Surf. Eng. Mater. Adv. Technol. 2012, 2012, 21428. [Google Scholar]
- Skočaj, M. Bacterial nanocellulose in papermaking. Cellulose 2019, 26, 6477–6488. [Google Scholar] [CrossRef]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of lignocellulosic biomass to nanocellulose: Structure and chemical process. Sci. World J. 2014, 2014, 631013. [Google Scholar] [CrossRef] [PubMed]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Shak, K.P.Y.; Pang, Y.L.; Mah, S.K. Nanocellulose: Recent advances and its prospects in environmental remediation. Beilstein J. Nanotechnol. 2018, 9, 2479–2498. [Google Scholar] [CrossRef] [PubMed]
- Jonoobi, M.; Mathew, A.P.; Oksman, K. Producing low-cost cellulose nanofiber from sludge as new source of raw materials. Ind. Crops Prod. 2012, 40, 232–238. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.P.; Grahn, M.; Mouzon, J.; Oksman, K. Nanoporous membranes with cellulose nanocrystals as functional entity in chitosan: Removal of dyes from water. Carbohydr. Polym. 2014, 112, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Karim, Z.; Mathew, A.P.; Kokol, V.; Wei, J.; Grahn, M. High-flux affinity membranes based on cellulose nanocomposites for removal of heavy metal ions from industrial effluents. RSC Adv. 2016, 6, 20644–20653. [Google Scholar] [CrossRef] [Green Version]
- Reshmy, R.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Binod, P.; Pandey, A. A green biorefinery platform for cost-effective nanocellulose production: Investigation of hydrodynamic properties and biodegradability of thin films. Biomass Convers. Biorefinery 2021, 11, 861–870. [Google Scholar] [CrossRef]
- Gupta, V.K.; Potumarthi, R.; O’Donovan, A.; Kubicek, C.P.; Sharma, G.D.; Tuohy, M.G. Chapter 2-Bioenergy Research: An Overview on Technological Developments and Bioresources. In Bioenergy Research: Advances and Applications; Gupta, V.K., Tuohy, M.G., Kubicek, C.P., Saddler, J., Xu, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 23–47. [Google Scholar]
- Di Blasi, C.; Branca, C.; Galgano, A. Biomass Screening for the Production of Furfural via Thermal Decomposition. Ind. Eng. Chem. Res. 2010, 49, 2658–2671. [Google Scholar] [CrossRef]
- Demirbaş, A.; Celik, A. Degradation of poplar and spruce wood chips using alkaline glycerol. Energy Sources 2005, 27, 1073–1084. [Google Scholar] [CrossRef]
- Rabemanolontsoa, H.; Saka, S. Comparative study on chemical composition of various biomass species. RSC Adv. 2013, 3, 3946–3956. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Hong, J. Supercritical CO2 pretreatment of lignocellulose enhances enzymatic cellulose hydrolysis. Bioresour. Technol. 2001, 77, 139–144. [Google Scholar] [CrossRef]
- Wang, S.; Lin, H.; Zhang, L.; Dai, G.; Zhao, Y.; Wang, X.; Ru, B. Structural Characterization and Pyrolysis Behavior of Cellulose and Hemicellulose Isolated from Softwood Pinus armandii Franch. Energy Fuels 2016, 30, 5721–5728. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Nagy, M.; Kim, D.H.; Eckert, C.A.; Hallett, J.P.; Liotta, C.L. From wood to fuels: Integrating biofuels and pulp production. Ind. Biotechnol. 2006, 2, 55–65. [Google Scholar] [CrossRef]
- Chen, L.; Fu, S. Enhanced Cellulase Hydrolysis of Eucalyptus Waste Fibers from Pulp Mill by Tween80-Assisted Ferric Chloride Pretreatment. J. Agric. Food. Chem. 2013, 61, 3293–3300. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, K.-H.; Ok, Y.S.; Jeon, Y.J.; Kwon, E.E. Pyrolysis of wastes generated through saccharification of oak tree by using CO2 as reaction medium. Appl. Therm. Eng. 2017, 110, 335–345. [Google Scholar] [CrossRef]
- Wypych, G. 7-Functional fillers–renewable and recycling. In Functional Fillers; Wypych, G., Ed.; ChemTec Publishing: Toronto, ON, Canada, 2018; pp. 181–195. [Google Scholar]
- Brosse, N.; El Hage, R.; Sannigrahi, P.; Ragauskas, A. Dilute sulphuric acid and ethanol organosolv pretreatment of miscanthus x giganteus. Cellul. Chem. Technol. 2010, 44, 71–78. [Google Scholar]
- Brylev, A.N.; Adylov, D.K.; Tukhtaeva, G.G.; Kamal’dinova, N.A.; Abidova, L.D.; Rakhimov, D.A. Polysaccharides of Rice Straw. Chem. Nat. Compd. 2001, 37, 569–570. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Dharmaraja, J.; Shobana, S.; Arvindnarayan, S.; Vadivel, M.; Atabani, A.E.; Pugazhendhi, A.; Kumar, G. Chapter 5-Biobutanol from lignocellulosic biomass: Bioprocess strategies. In Lignocellulosic Biomass to Liquid Biofuels; Yousuf, A., Pirozzi, D., Sannino, F., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 169–193. [Google Scholar]
- Deshavath, N.N.; Veeranki, V.D.; Goud, V.V. Chapter 1-Lignocellulosic feedstocks for the production of bioethanol: Availability, structure, and composition. In Sustainable Bioenergy; Rai, M., Ingle, A.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–19. [Google Scholar]
- Kumar, A.; Gautam, A.; Dutt, D. Biotechnological transformation of lignocellulosic biomass in to industrial products: An overview. Adv. Biosci. Biotechnol. 2016, 7, 149–168. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, Ó.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef]
- Boneberg, B.S.; Machado, G.D.; Santos, D.F.; Gomes, F.; Faria, D.J.; Gomes, L.A.; Santos, F.A. Biorefinery of lignocellulosic biopolymers. Rev. Electron. Cient. UERGS 2016, 1, 79–100. [Google Scholar] [CrossRef] [Green Version]
- Deepa, B.; Abraham, E.; Cherian, B.M.; Bismarck, A.; Blaker, J.J.; Pothan, L.A.; Leao, A.L.; de Souza, S.F.; Kottaisamy, M. Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion. Bioresour. Technol. 2011, 102, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues–Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Zhou, Y.; Li, Y. Liquid hot water and alkaline pretreatment of soybean straw for improving cellulose digestibility. Bioresour. Technol. 2011, 102, 6254–6259. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, R.A.; Chen, Y.; Sharma-Shivappa, R.R.; Boyette, M.D.; Osborne, J. A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour. Technol. 2007, 98, 3000–3011. [Google Scholar] [CrossRef]
- Aswathy, U.S.; Sukumaran, R.K.; Devi, G.L.; Rajasree, K.P.; Singhania, R.R.; Pandey, A. Bio-ethanol from water hyacinth biomass: An evaluation of enzymatic saccharification strategy. Bioresour. Technol. 2010, 101, 925–930. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M. Microcrystalline cellulose from Posidonia oceanica brown algae: Extraction and characterization. Int. J. Biol. Macromol. 2019, 138, 837–845. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Feitosa, J.P.A.; da Gama, F.M.P.; Morais, J.P.S.; Andrade, F.K.; de Souza Filho, M.d.S.M.; Rosa, M.d.F. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Byrd, C.S.; Barlaz, M.A. Anaerobic biodegradability of cellulose and hemicellulose in excavated refuse samples using a biochemical methane potential assay. J. Ind. Microbiol. 1994, 13, 147–153. [Google Scholar] [CrossRef]
- Dai, H.; Ou, S.; Huang, Y.; Huang, H. Utilization of pineapple peel for production of nanocellulose and film application. Cellulose 2018, 25, 1743–1756. [Google Scholar] [CrossRef]
- Chang, P.S.; Robyt, J.F. Oxidation of primary alcohol groups of naturally occurring polysaccharides with 2, 2, 6, 6-tetramethyl-1-piperidine oxoammonium ion. J. Carbohydr. Chem. 1996, 15, 819–830. [Google Scholar] [CrossRef]
- Dufresne, A.; Cavaillé, J.Y.; Vignon, M.R. Mechanical behavior of sheets prepared from sugar beet cellulose microfibrils. J. Appl. Polym. Sci. 1997, 64, 1185–1194. [Google Scholar] [CrossRef]
- Stelte, W.; Sanadi, A.R. Preparation and characterization of cellulose nanofibers from two commercial hardwood and softwood pulps. Ind. Eng. Chem. Res. 2009, 48, 11211–11219. [Google Scholar] [CrossRef]
- Tan, X.Y.; Abd Hamid, S.B.; Lai, C.W. Preparation of high crystallinity cellulose nanocrystals (CNCs) by ionic liquid solvolysis. Biomass Bioenergy 2015, 81, 584–591. [Google Scholar] [CrossRef]
- Islam, M.T.; Alam, M.M.; Patrucco, A.; Montarsolo, A.; Zoccola, M. Preparation of nanocellulose: A review. AATCC J. Res. 2014, 1, 17–23. [Google Scholar] [CrossRef]
- Nishi, Y.; Uryu, M.; Yamanaka, S.; Watanabe, K.; Kitamura, N.; Iguchi, M.; Mitsuhashi, S. The structure and mechanical properties of sheets prepared from bacterial cellulose. J. Mater. Sci. 1990, 25, 2997–3001. [Google Scholar] [CrossRef]
- Jahanbaani, A.R.; Behzad, T.; Borhani, S.; Darvanjooghi, M.H.K. Electrospinning of cellulose nanofibers mat for laminated epoxy composite production. Fibers Polym. 2016, 17, 1438–1448. [Google Scholar] [CrossRef]
- Kim, C.-W.; Kim, D.-S.; Kang, S.-Y.; Marquez, M.; Joo, Y.L. Structural studies of electrospun cellulose nanofibers. Polymer 2006, 47, 5097–5107. [Google Scholar] [CrossRef]
- Ziolkowska, J.R. Desalination leaders in the global market–current trends and future perspectives. Water Sci. Technol. Water Supply 2016, 16, 563–578. [Google Scholar] [CrossRef]
- Alhumoud, J.M.; Al-Humaidi, H.; Al-Ghusain, I.N.; Alhumoud, A.M. Cost/benefit evaluation of sulaibiya wastewater treatment plant in Kuwait. Int. Bus. Econ. Res. J. IBER 2010, 9, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Gu, J.; Wang, S.; Zhang, M.; Liu, Y. Performance, membrane fouling control and cost analysis of an integrated anaerobic fixed-film MBR and reverse osmosis process for municipal wastewater reclamation to NEWater-like product water. J. Membr. Sci. 2020, 593, 117442. [Google Scholar] [CrossRef]
- Bazaluk, O.; Havrysh, V.; Nitsenko, V.; Baležentis, T.; Streimikiene, D.; Tarkhanova, E.A. Assessment of green methanol production potential and related economic and environmental benefits: The case of China. Energies 2020, 13, 3113. [Google Scholar] [CrossRef]
- Li, L.; Shi, W.; Yu, S. Research on forward osmosis membrane technology still needs improvement in water recovery and wastewater treatment. Water 2020, 12, 107. [Google Scholar] [CrossRef] [Green Version]
- Webley, J. Technology developments in forward osmosis to address water purification. Desalination Water Treat. 2015, 55, 2612–2617. [Google Scholar] [CrossRef]
- Suryaprabha, T.; Sethuraman, M.G. Fabrication of copper-based superhydrophobic self-cleaning antibacterial coating over cotton fabric. Cellulose 2017, 24, 395–407. [Google Scholar] [CrossRef]
- Bai, X.; Shen, Y.; Tian, H.; Yang, Y.; Feng, H.; Li, J. Facile fabrication of superhydrophobic wood slice for effective water-in-oil emulsion separation. Sep. Purif. Technol. 2019, 210, 402–408. [Google Scholar] [CrossRef]
- Sanguanwong, A.; Pavasant, P.; Jarunglumlert, T.; Nakagawa, K.; Flood, A.; Prommuak, C. Hydrophobic cellulose aerogel from waste napkin paper for oil sorption applications. Nord. Pulp Pap. Res. J. 2020, 35, 137–147. [Google Scholar] [CrossRef]
- Ebert, D.; Bhushan, B. Transparent, superhydrophobic, and wear-resistant surfaces using deep reactive ion etching on PDMS substrates. J. Colloid Interface Sci. 2016, 481, 82–90. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, D.H.; Kim, Y.D. Superhydrophobic, flexible and gas-permeable membrane prepared by a simple one-step vapor deposition. Korean J. Chem. Eng. 2016, 33, 1743–1748. [Google Scholar] [CrossRef]
- Pacifico, J.; Endo, K.; Morgan, S.; Mulvaney, P. Superhydrophobic effects of self-assembled monolayers on micropatterned surfaces: 3-D arrays mimicking the lotus leaf. Langmuir 2006, 22, 11072–11076. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.K.; Bal, K.; He, F.; Fan, J. Design of an outstanding super-hydrophobic surface by electro-spinning. Appl. Surf. Sci. 2011, 257, 7003–7009. [Google Scholar] [CrossRef]
- Shi, Y.; Xiao, X. Facile spray-coating for fabrication of superhydrophobic SiO2/PVDF nanocomposite coating on paper surface. J. Dispersion Sci. Technol. 2016, 37, 640–645. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, F.; Yu, X.; Liu, H.; Fu, Y.; Wang, Z.; Jiang, L.; Li, X. Polyelectrolyte multilayer as matrix for electrochemical deposition of gold clusters: Toward super-hydrophobic surface. J. Am. Chem. Soc. 2004, 126, 3064–3065. [Google Scholar] [CrossRef]
- Choudhury, R.R.; Sahoo, S.K.; Gohil, J.M. Potential of bioinspired cellulose nanomaterials and nanocomposite membranes thereof for water treatment and fuel cell applications. Cellulose 2020, 27, 6719–6746. [Google Scholar] [CrossRef]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Wu, J.; Qiu, Q.; Wang, Y.; Zhang, H.; Qin, X. Asymmetric water affinity on antibacterial electrospun sub-micro cellulose acetate Janus membrane. Mater. Lett. 2019, 256, 126607. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Kettunen, M.; Ras, R.H.A.; Ikkala, O. Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. ACS Appl. Mater. Interfaces 2011, 3, 1813–1816. [Google Scholar] [CrossRef]
- Manca, M.; Cannavale, A.; De Marco, L.; Arico, A.S.; Cingolani, R.; Gigli, G. Durable superhydrophobic and antireflective surfaces by trimethylsilanized silica nanoparticles-based sol− gel processing. Langmuir 2009, 25, 6357–6362. [Google Scholar] [CrossRef]
- Salam, A.; Lucia, L.A.; Jameel, H. Fluorine-based surface decorated cellulose nanocrystals as potential hydrophobic and oleophobic materials. Cellulose 2015, 22, 397–406. [Google Scholar] [CrossRef]
- Shang, W.; Huang, J.; Luo, H.; Chang, P.R.; Feng, J.; Xie, G. Hydrophobic modification of cellulose nanocrystal via covalently grafting of castor oil. Cellulose 2013, 20, 179–190. [Google Scholar] [CrossRef]
- Song, J.; Rojas, O.J. Paper chemistry: Approaching super-hydrophobicity from cellulosic materials: A review. Nord. Pulp Pap. Res. J. 2013, 28, 216–238. [Google Scholar] [CrossRef]
- Li, S.; Xie, H.; Zhang, S.; Wang, X. Facile transformation of hydrophilic cellulose into superhydrophobic cellulose. Chem. Commun. 2007, 46, 4857–4859. [Google Scholar] [CrossRef] [PubMed]
- Young, T., III. An essay on the cohesion of fluids. Philos. Trans. R. Soc. London 1805, 95, 65–87. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Prog. Mater Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhao, Y.; Xu, L.; Gao, X.; Zheng, Y.; Jiang, L. How does the leaf margin make the lotus surface dry as the lotus leaf floats on water? Soft Matter 2008, 4, 2232–2237. [Google Scholar] [CrossRef]
- Su, B.; Tian, Y.; Jiang, L. Bioinspired interfaces with superwettability: From materials to chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. [Google Scholar] [CrossRef]
- Ma, Q.; Yu, Y.; Sindoro, M.; Fane, A.G.; Wang, R.; Zhang, H. Carbon-based functional materials derived from waste for water remediation and energy storage. Adv. Mater. 2017, 29, 1605361. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Kota, A.K.; Kwon, G.; Choi, W.; Mabry, J.M.; Tuteja, A. Hygro-responsive membranes for effective oil–water separation. Nat. Commun. 2012, 3, 1025. [Google Scholar] [CrossRef] [Green Version]
- Bae, G.Y.; Min, B.G.; Jeong, Y.G.; Lee, S.C.; Jang, J.H.; Koo, G.H. Superhydrophobicity of cotton fabrics treated with silica nanoparticles and water-repellent agent. J. Colloid Interface Sci. 2009, 337, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.-H.; Jia, S.-T.; Zhang, J.; Ma, J.-Z. Large-area fabrication of superhydrophobic surfaces for practical applications: An overview. Sci. Technol. Adv. Mater. 2010, 11, 033002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, P.; Wang, M.; Zhao, H.; Yang, J.; Xu, F. Sustainable, reusable, and superhydrophobic aerogels from microfibrillated cellulose for highly effective oil/water separation. ACS Sustain. Chem. Eng. 2016, 4, 6409–6416. [Google Scholar] [CrossRef]
- Laitinen, O.; Suopajarvi, T.; Osterberg, M.; Liimatainen, H. Hydrophobic, superabsorbing aerogels from choline chloride-based deep eutectic solvent pretreated and silylated cellulose nanofibrils for selective oil removal. ACS Appl. Mater. Interfaces 2017, 9, 25029–25037. [Google Scholar] [CrossRef] [Green Version]
- Fan, T.; Qian, Q.; Hou, Z.; Liu, Y.; Lu, M. Preparation of smart and reversible wettability cellulose fabrics for oil/water separation using a facile and economical method. Carbohydr. Polym. 2018, 200, 63–71. [Google Scholar] [CrossRef]
- Zheng, X.; Fu, S. Reconstructing micro/nano hierarchical structures particle with nanocellulose for superhydrophobic coatings. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 171–179. [Google Scholar] [CrossRef]
- Roy, S.; Zhai, L.; Van Hai, L.; Kim, J.W.; Park, J.H.; Kim, H.C.; Kim, J. One-step nanocellulose coating converts tissue paper into an efficient separation membrane. Cellulose 2018, 25, 4871–4886. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S. Remodeling of raw cotton fiber into flexible, squeezing-resistant macroporous cellulose aerogel with high oil retention capability for oil/water separation. Sep. Purif. Technol. 2019, 221, 303–310. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Li, H.; Jing, X.; Zhang, Q.; Feng, P.-Y.; He, P.; Liu, Y. Superhydrophobic cellulose nanofibril/silica fiber/Fe3O4 nanocomposite aerogel for magnetically driven selective oil absorption. Cellulose 2020, 27, 8909–8922. [Google Scholar] [CrossRef]
- Pu, Y.; Yang, J.; Russell, S.J.; Ning, X. Cotton nonwovens with unidirectional water-transport properties produced by atmospheric plasma deposition. Cellulose 2021, 28, 4427–4438. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, C.; Cui, S.; Wu, X.; Jiang, S. Microstructure Characterization and Oil Absorption Performance of Superhydrophobic Cotton Cellulose Aerogel. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2021, 36, 538–545. [Google Scholar] [CrossRef]
- Dilamian, M.; Noroozi, B. Rice straw agri-waste for water pollutant adsorption: Relevant mesoporous super hydrophobic cellulose aerogel. Carbohydr. Polym. 2021, 251, 117016. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A. Nanocellulose processing properties and potential applications. Curr. For. Rep. 2019, 5, 76–89. [Google Scholar] [CrossRef]
- Wang, S.; Wang, F.; Song, Z.; Song, X.; Yang, X.; Zhan, Q. Preparation of Cellulose Nanocrystals Using Highly Recyclable Organic Acid Treated Softwood Pulp. BioResources 2019, 14, 9331–9351. [Google Scholar] [CrossRef]
- Hokkanen, S.; Repo, E.; Lou, S.; Sillanpää, M. Removal of arsenic (V) by magnetic nanoparticle activated microfibrillated cellulose. Chem. Eng. J. 2015, 260, 886–894. [Google Scholar] [CrossRef]
- Fowler, M.; Andrews, J.E.; Brimblecombe, P.; Jickells, T.D.; Liss, P.S.; Reid, B.J. An Introduction to Environmental Chemistry, xxi + 298 pp. Oxford: Blackwell Science. Price£ 24.99 (paperback). ISBN 0 632 05905 2. Geol. Mag. 2004, 141, 748–749. [Google Scholar] [CrossRef]
- Edition, F. Guidelines for drinking-water quality. WHO Chron. 2011, 38, 104–108. [Google Scholar]
- World Health Organization. Chloride in Drinking-Water. "Guidelines for Drinking-Water Quality"; World Health Organization: Geneva, Switzerland, 2011; Volume 216, pp. 303–304. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives; World Health Organization. Evaluation of Certain Food Additives and Contaminants: Fifty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- World Health Organization. Chromium in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality. 2003. Available online: https://www.who.int/water_sanitation_health/dwq/chromium.pdf (accessed on 12 August 2021).
- World Health Organization. Lead in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- World Health Organization. Zinc in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality. 2003. Available online: https://www.who.int/water_sanitation_health/dwq/chemicals/zinc.pdf (accessed on 12 August 2021).
- World Health Organization. Nitrate and Nitrite in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Zhang, N.; Zang, G.-L.; Shi, C.; Yu, H.-Q.; Sheng, G.-P. A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal. J. Hazard. Mater. 2016, 316, 11–18. [Google Scholar] [CrossRef]
- Mo, L.; Pang, H.; Lu, Y.; Li, Z.; Kang, H.; Wang, M.; Zhang, S.; Li, J. Wood-inspired nanocellulose aerogel adsorbents with excellent selective pollutants capture, superfast adsorption, and easy regeneration. J. Hazard. Mater. 2021, 415, 125612. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Cha, B.G.; Kim, J. Three-dimensional macroporous alginate scaffolds embedded with akaganeite nanorods for the filter-based high-speed preparation of arsenic-free drinking water. ACS Appl. Nano Mater. 2018, 1, 1940–1948. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, G.; Wang, P. Visualized fibrous adsorbent prepared by the microwave-assisted method for both detection and removal of heavy metal ions. ACS Sustain. Chem. Eng. 2018, 7, 1159–1168. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, H.; Chen, S.; Long, F.; Huang, B.; Yang, B.; Pan, X. A magnetically recyclable chitosan composite adsorbent functionalized with EDTA for simultaneous capture of anionic dye and heavy metals in complex wastewater. Chem. Eng. J. 2019, 356, 69–80. [Google Scholar] [CrossRef]
- Pu, S.; Hou, Y.; Yan, C.; Ma, H.; Huang, H.; Shi, Q.; Mandal, S.; Diao, Z.; Chu, W. In situ coprecipitation formed highly water-dispersible magnetic chitosan nanopowder for removal of heavy metals and its adsorption mechanism. ACS Sustain. Chem. Eng. 2018, 6, 16754–16765. [Google Scholar] [CrossRef]
- Das, S.; Chakraborty, P.; Ghosh, R.; Paul, S.; Mondal, S.; Panja, A.; Nandi, A.K. Folic acid-polyaniline hybrid hydrogel for adsorption/reduction of chromium (VI) and selective adsorption of anionic dye from water. ACS Sustain. Chem. Eng. 2017, 5, 9325–9337. [Google Scholar] [CrossRef]
- Li, C.; Yan, Y.; Zhang, Q.; Zhang, Z.; Huang, L.; Zhang, J.; Xiong, Y.; Tan, S. Adsorption of Cd2+ and Ni2+ from aqueous single-metal solutions on graphene oxide-chitosan-poly (vinyl alcohol) hydrogels. Langmuir 2019, 35, 4481–4490. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Wang, Q.; Di, J.; Miao, S.; Yu, J. Amino-functionalized porous nanofibrous membranes for simultaneous removal of oil and heavy-metal ions from wastewater. ACS Appl. Mater. Interfaces 2018, 11, 1672–1679. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, C.; Huang, A. EDTA-functionalized covalent organic framework for the removal of heavy-metal ions. ACS Appl. Mater. Interfaces 2019, 11, 32186–32191. [Google Scholar] [CrossRef]
- Sehaqui, H.; de Larraya, U.P.; Liu, P.; Pfenninger, N.; Mathew, A.P.; Zimmermann, T.; Tingaut, P. Enhancing adsorption of heavy metal ions onto biobased nanofibers from waste pulp residues for application in wastewater treatment. Cellulose 2014, 21, 2831–2844. [Google Scholar] [CrossRef]
- Hu, Z.-H.; Omer, A.M.; Ouyang, X.k.; Yu, D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zuo, K.; Wu, W.; Xu, Z.; Yi, Y.; Jing, Y.; Dai, H.; Fang, G. Shape memory aerogels from nanocellulose and polyethyleneimine as a novel adsorbent for removal of Cu(II) and Pb(II). Carbohydr. Polym. 2018, 196, 376–384. [Google Scholar] [CrossRef]
- Singh, K.; Arora, J.K.; Sinha, T.J.M.; Srivastava, S. Functionalization of nanocrystalline cellulose for decontamination of Cr(III) and Cr(VI) from aqueous system: Computational modeling approach. Clean Technol. Environ. Policy 2014, 16, 1179–1191. [Google Scholar] [CrossRef]
- Alipour, A.; Zarinabadi, S.; Azimi, A.; Mirzaei, M. Adsorptive removal of Pb(II) ions from aqueous solutions by thiourea-functionalized magnetic ZnO/nanocellulose composite: Optimization by response surface methodology (RSM). Int. J. Biol. Macromol. 2020, 151, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Bisla, V.; Rattan, G.; Singhal, S.; Kaushik, A. Green and novel adsorbent from rice straw extracted cellulose for efficient adsorption of Hg (II) ions in an aqueous medium. Int. J. Biol. Macromol. 2020, 161, 194–203. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Mutesse, B.; Leswifi, T.Y.; Onyango, M.S. Highly efficient removal of nickel and cadmium from water using sawdust-derived cellulose nanocrystals. J. Environ. Chem. Eng. 2019, 7, 103251. [Google Scholar] [CrossRef]
- Abiaziem, C.; Williams, A.; Inegbenebor, A.; Onwordi, C.; Ehi-Eromosele, C.; Petrik, L. Adsorption of lead ion from aqueous solution unto cellulose nanocrystal from cassava peel. J. Phys. Conf. Ser. 2019, 1299, 012122. [Google Scholar] [CrossRef]
- Chai, F.; Wang, R.; Yan, L.; Li, G.; Cai, Y.; Xi, C. Facile fabrication of pH-sensitive nanoparticles based on nanocellulose for fast and efficient As(V) removal. Carbohydr. Polym. 2020, 245, 116511. [Google Scholar] [CrossRef]
- Kaur, M.; Kumari, S.; Sharma, P. Removal of Pb (II) from aqueous solution using nanoadsorbent of Oryza sativa husk: Isotherm, kinetic and thermodynamic studies. Biotechnol. Rep. 2020, 25, e00410. [Google Scholar] [CrossRef]
- Dong, F.; Xu, X.; Shaghaleh, H.; Guo, J.; Guo, L.; Qian, Y.; Liu, H.; Wang, S. Factors influencing the morphology and adsorption performance of cellulose nanocrystal/iron oxide nanorod composites for the removal of arsenic during water treatment. Int. J. Biol. Macromol. 2020, 156, 1418–1424. [Google Scholar] [CrossRef]
- Park, S.-H.; Shin, S.S.; Park, C.H.; Jeon, S.; Gwon, J.; Lee, S.-Y.; Kim, S.-J.; Kim, H.-J.; Lee, J.-H. Poly(acryloyl hydrazide)-grafted cellulose nanocrystal adsorbents with an excellent Cr(VI) adsorption capacity. J. Hazard. Mater. 2020, 394, 122512. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, H.; Deutschman, C.; Yang, T.; Tam, K.C. Novel design of Fe-Cu alloy coated cellulose nanocrystals with strong antibacterial ability and efficient Pb2+ removal. Carbohydr. Polym. 2020, 234, 115889. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Z.Y.; Su, X.P.; Chen, X.; Jiang, L.; Yao, J.M. Adsorption removal of dyes from single and binary solutions using a cellulose-based bioadsorbent. ACS Sustain. Chem. Eng. 2015, 3, 432–442. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Batmaz, R.; Mohammed, N.; Zaman, M.; Minhas, G.; Berry, R.M.; Tam, K.C. Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 2014, 21, 1655–1665. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Zhang, D.-Z.; Lu, F.-F.; Yao, J. New approach for single-step extraction of carboxylated cellulose nanocrystals for their use as adsorbents and flocculants. ACS Sustain. Chem. Eng. 2016, 4, 2632–2643. [Google Scholar] [CrossRef]
- Manna, S.; Roy, D.; Saha, P.; Gopakumar, D.; Thomas, S. Rapid methylene blue adsorption using modified lignocellulosic materials. Process Saf. Environ. Prot. 2017, 107, 346–356. [Google Scholar] [CrossRef]
- Gopakumar, D.A.; Pasquini, D.; Henrique, M.A.; de Morais, L.C.; Grohens, Y.; Thomas, S. Meldrum’s acid modified cellulose nanofiber-based polyvinylidene fluoride microfiltration membrane for dye water treatment and nanoparticle removal. ACS Sustain. Chem. Eng. 2017, 5, 2026–2033. [Google Scholar] [CrossRef]
- Wang, J.-J.; Yang, H.-C.; Wu, M.-B.; Zhang, X.; Xu, Z.-K. Nanofiltration membranes with cellulose nanocrystals as an interlayer for unprecedented performance. J. Mater. Chem. A 2017, 5, 16289–16295. [Google Scholar] [CrossRef]
- Zheng, X.; Li, X.; Li, J.; Wang, L.; Jin, W.; Pei, Y.; Tang, K. Efficient removal of anionic dye (Congo red) by dialdehyde microfibrillated cellulose/chitosan composite film with significantly improved stability in dye solution. Int. J. Biol. Macromol. 2018, 107, 283–289. [Google Scholar] [CrossRef]
- Al-Ahmed, Z.A.; Hassan, A.A.; El-Khouly, S.M.; El-Shafey, S.E. TEMPO-oxidized cellulose nanofibers/TiO2 nanocomposite as new adsorbent for Brilliant Blue dye removal. Polym. Bull. 2020, 77, 6213–6226. [Google Scholar] [CrossRef]
- Pinto, A.H.; Taylor, J.K.; Chandradat, R.; Lam, E.; Liu, Y.; Leung, A.C.W.; Keating, M.; Sunasee, R. Wood-based cellulose nanocrystals as adsorbent of cationic toxic dye, Auramine O, for water treatment. J. Environ. Chem. Eng. 2020, 8, 104187. [Google Scholar] [CrossRef]
- Tavakolian, M.; Wiebe, H.; Sadeghi, M.A.; van de Ven, T.G.M. Dye removal using hairy nanocellulose: Experimental and theoretical investigations. ACS Appl. Mater. Interfaces 2019, 12, 5040–5049. [Google Scholar] [CrossRef] [PubMed]
- Oyewo, O.A.; Adeniyi, A.; Sithole, B.B.; Onyango, M.S. Sawdust-based cellulose nanocrystals incorporated with ZnO nanoparticles as efficient adsorption media in the removal of methylene blue dye. ACS Omega 2020, 5, 18798–18807. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, J.; Lin, S.; Xiao, H.; Yang, Q.; Chen, S.; Yan, B.; Gu, Y. Environmentally friendly nanocomposites based on cellulose nanocrystals and polydopamine for rapid removal of organic dyes in aqueous solution. Cellulose 2020, 27, 2085–2097. [Google Scholar] [CrossRef]
- Arabkhani, P.; Asfaram, A. Development of a novel three-dimensional magnetic polymer aerogel as an efficient adsorbent for malachite green removal. J. Hazard. Mater. 2020, 384, 121394. [Google Scholar] [CrossRef]
- Ranjbar, D.; Raeiszadeh, M.; Lewis, L.; MacLachlan, M.J.; Hatzikiriakos, S.G. Adsorptive removal of Congo red by surfactant modified cellulose nanocrystals: A kinetic, equilibrium, and mechanistic investigation. Cellulose 2020, 27, 3211–3232. [Google Scholar] [CrossRef]
- Mutar, H.R.; Jasim, K.K. Adsorption study of disperse yellow dye on nanocellulose surface. Mater. Today Proc. 2021, 1–7. [Google Scholar] [CrossRef]
- Tsade Kara, H.; Anshebo, S.T.; Sabir, F.K.; Adam Workineh, G. Removal of Methylene Blue Dye from Wastewater Using Periodiated Modified Nanocellulose. Int. J. Chem. Eng. 2021, 2021, 9965452. [Google Scholar] [CrossRef]
- Shahnaz, T.; Bedadeep, D.; Narayanasamy, S. Investigation of the adsorptive removal of methylene blue using modified nanocellulose. Int. J. Biol. Macromol. 2022, 200, 162–171. [Google Scholar] [CrossRef]
- Ariaeenejad, S.; Motamedi, E.; Salekdeh, G.H. Highly efficient removal of dyes from wastewater using nanocellulose from quinoa husk as a carrier for immobilization of laccase. Bioresour. Technol. 2022, 349, 126833. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Milletto, C.; Monti, S.; Zhu, C.; Mathew, A.P. Design of ultrathin hybrid membranes with improved retention efficiency of molecular dyes. RSC Adv. 2019, 9, 28657–28669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Nanocellulose-Enabled Membranes for Water Purification: Perspectives. Adv. Sustain. Syst. 2020, 4, 1900114. [Google Scholar] [CrossRef]
- Suwaileh, W.; Pathak, N.; Shon, H.; Hilal, N. Forward osmosis membranes and processes: A comprehensive review of research trends and future outlook. Desalination 2020, 485, 114455. [Google Scholar] [CrossRef]
- Elkony, Y.; Mansour, E.S.; Elhusseiny, A.; Ebrahim, S. Effect of cellulose acetate/cellulose triacetate ratio on reverse osmosis blend membrane performance. Polym. Eng. Sci. 2020, 60, 2852–2863. [Google Scholar] [CrossRef]
- Ounifi, I.; Guesmi, Y.; Ursino, C.; Castro-Muñoz, R.; Agougui, H.; Jabli, M.; Hafiane, A.; Figoli, A.; Ferjani, E. Synthesis and Characterization of a Thin-Film Composite Nanofiltration Membrane Based on Polyamide-Cellulose Acetate: Application for Water Purification. J. Polym. Environ. 2021, 30, 707–718. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.; Zhao, Y.; Zha, F.; Wang, Q.; Lei, Z. Correction: One-step fabrication of robust fabrics with both-faced superhydrophobicity for the separation and capture of oil from water. PCCP 2015, 17, 11112. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.-Y.; Wang, W.; Mao, L.-H.; Wang, C.-F.; Chen, S. Versatile superhydrophobic and photocatalytic films generated from TiO 2–SiO 2@ PDMS and their applications on fabrics. J. Mater. Chem. A 2014, 2, 4178–4184. [Google Scholar] [CrossRef]
- Gao, J.; Huang, X.; Xue, H.; Tang, L.; Li, R.K.Y. Facile preparation of hybrid microspheres for super-hydrophobic coating and oil-water separation. Chem. Eng. J. 2017, 326, 443–453. [Google Scholar] [CrossRef]
- Cai, H.; Mu, W.; Liu, W.; Zhang, X.; Deng, Y. Sol–gel synthesis highly porous titanium dioxide microspheres with cellulose nanofibrils-based aerogel templates. Inorg. Chem. Commun. 2015, 51, 71–74. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, H.; Tan, S.; Jiang, X.; Wu, W.; Shi, J.; Chen, P. Ultralight super-hydrophobic carbon aerogels based on cellulose nanofibers/poly (vinyl alcohol)/graphene oxide (CNFs/PVA/GO) for highly effective oil–water separation. Beilstein J. Nanotechnol. 2018, 9, 508–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Zhao, H.; Li, X.; Su, D.; Zhang, F.; Ji, H.; Liu, R. Superelastic and superhydrophobic bacterial cellulose/silica aerogels with hierarchical cellular structure for oil absorption and recovery. J. Hazard. Mater. 2018, 346, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Sobhana, S.S.L.; Zhang, X.; Kesavan, L.; Liias, P.; Fardim, P. Layered double hydroxide interfaced stearic acid–Cellulose fibres: A new class of super-hydrophobic hybrid materials. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 416–424. [Google Scholar] [CrossRef]
- Li, P.; Zeng, L.; Gao, J.; Yao, L.; Zhao, X.; Wu, Q.; Liu, X.; Wang, Y.; Yang, X.; Shi, J. Perturbation of normal algal growth by black phosphorus nanosheets: The role of degradation. Environ. Sci. Technol. Lett. 2019, 7, 35–41. [Google Scholar] [CrossRef]
- Hou, Y.; Duan, C.; Zhu, G.; Luo, H.; Liang, S.; Jin, Y.; Zhao, N.; Xu, J. Functional bacterial cellulose membranes with 3D porous architectures: Conventional drying, tunable wettability and water/oil separation. J. Membr. Sci. 2019, 591, 117312. [Google Scholar] [CrossRef]
- Cheng, R.; Kang, M.; Zhuang, S.; Shi, L.; Zheng, X.; Wang, J. Adsorption of Sr (II) from water by mercerized bacterial cellulose membrane modified with EDTA. J. Hazard. Mater. 2019, 364, 645–653. [Google Scholar] [CrossRef]
- Yu, Y.; Shi, X.; Liu, L.; Yao, J. Highly compressible and durable superhydrophobic cellulose aerogels for oil/water emulsion separation with high flux. J. Mater. Sci. 2021, 56, 2763–2776. [Google Scholar] [CrossRef]
- Shi, C.; Chen, Y.; Yu, Z.; Li, S.; Chan, H.; Sun, S.; Chen, G.; He, M.; Tian, J. Sustainable and superhydrophobic spent coffee ground-derived holocellulose nanofibers foam for continuous oil/water separation. Sustain. Mater. Technol. 2021, 28, e00277. [Google Scholar] [CrossRef]
- Akhlamadi, G.; Goharshadi, E.K. Sustainable and superhydrophobic cellulose nanocrystal-based aerogel derived from waste tissue paper as a sorbent for efficient oil/water separation. Process Saf. Environ. Prot. 2021, 154, 155–167. [Google Scholar] [CrossRef]
- Wang, L.; Hui, L.; Su, W. Superhydrophobic modification of nanocellulose based on an octadecylamine/dopamine system. Carbohydr. Polym. 2022, 275, 118710. [Google Scholar] [CrossRef]
- Qiao, A.; Huang, R.; Penkova, A.; Qi, W.; He, Z.; Su, R. Superhydrophobic, elastic and anisotropic cellulose nanofiber aerogels for highly effective oil/water separation. Sep. Purif. Technol. 2022, 295, 121266. [Google Scholar] [CrossRef]
- Dong, L.; Zhao, Y. CO 2-switchable membranes: Structures, functions, and separation applications in aqueous medium. J. Mater. Chem. A 2020, 8, 16738–16746. [Google Scholar] [CrossRef]
- Tong, Y.; He, M.; Zhou, Y.; Nie, S.; Zhong, X.; Fan, L.; Huang, T.; Liao, Q.; Wang, Y. Three-Dimensional Hierarchical Architecture of the TiO2/Ti3C2T x/RGO Ternary Composite Aerogel for Enhanced Electromagnetic Wave Absorption. ACS Sustain. Chem. Eng. 2018, 6, 8212–8222. [Google Scholar] [CrossRef]
- Yang, H.C.; Hou, J.; Chen, V.; Xu, Z.K. Janus membranes: Exploring duality for advanced separation. Angew. Chem. Int. Ed. 2016, 55, 13398–13407. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Surface segregation of fluorinated modifying macromolecule for hydrophobic/hydrophilic membrane preparation and application in air gap and direct contact membrane distillation. J. Membr. Sci. 2012, 417, 163–173. [Google Scholar] [CrossRef]
- Lv, Y.; Li, Q.; Hou, Y.; Wang, B.; Zhang, T. Facile preparation of an asymmetric wettability Janus cellulose membrane for switchable emulsions’ separation and antibacterial property. ACS Sustain. Chem. Eng. 2019, 7, 15002–15011. [Google Scholar] [CrossRef]
- Duolikun, T.; Ghazali, N.; Leo, B.F.; Lee, H.V.; Lai, C.W.; Johan, M.R.B. Asymmetric Cellulosic Membranes: Current and Future Aspects. Symmetry 2020, 12, 1160. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Liu, Y.; Xue, C.; Wang, Y.; Dai, J. Preparation of Janus membrane based on biomimetic polydopamine interface regulation and superhydrophobic attapulgite spraying for on-demand oil-water emulsion separation. J. Membr. Sci. 2021, 627, 119242. [Google Scholar] [CrossRef]
- Agaba, A.; Marriam, I.; Tebyetekerwa, M.; Yuanhao, W. Janus hybrid sustainable all-cellulose nanofiber sponge for oil-water separation. Int. J. Biol. Macromol. 2021, 185, 997–1004. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Q.; Gao, M.; Zhou, N.; Shi, J.; Jiang, W. Fabrication of Janus cellulose nanocomposite membrane for various water/oil separation and selective one-way transmission. J. Environ. Chem. Eng. 2021, 9, 106016. [Google Scholar] [CrossRef]
- Singh, N.; Balasubramanian, K. An effective technique for removal and recovery of uranium (VI) from aqueous solution using cellulose–camphor soot nanofibers. RSC Adv. 2014, 4, 27691–27701. [Google Scholar] [CrossRef]
- Zhou, W.; He, J.; Cui, S.; Gao, W. Preparation of electrospun silk fibroin/Cellulose Acetate blend nanofibers and their applications to heavy metal ions adsorption. Fibers Polym. 2011, 12, 431–437. [Google Scholar] [CrossRef]
- Stephen, M.; Catherine, N.; Brenda, M.; Andrew, K.; Leslie, P.; Corrine, G. Oxolane-2, 5-dione modified electrospun cellulose nanofibers for heavy metals adsorption. J. Hazard. Mater. 2011, 192, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Goetz, L.A.; Jalvo, B.; Rosal, R.; Mathew, A.P. Superhydrophilic anti-fouling electrospun cellulose acetate membranes coated with chitin nanocrystals for water filtration. J. Membr. Sci. 2016, 510, 238–248. [Google Scholar] [CrossRef]
- Taha, A.A.; Wu, Y.-n.; Wang, H.; Li, F. Preparation and application of functionalized cellulose acetate/silica composite nanofibrous membrane via electrospinning for Cr (VI) ion removal from aqueous solution. J. Environ. Manag. 2012, 112, 10–16. [Google Scholar] [CrossRef]
- Cai, J.; Lei, M.; Zhang, Q.; He, J.-R.; Chen, T.; Liu, S.; Fu, S.-H.; Li, T.-T.; Liu, G.; Fei, P. Electrospun composite nanofiber mats of Cellulose@ Organically modified montmorillonite for heavy metal ion removal: Design, characterization, evaluation of absorption performance. Compos. Part A Appl. Sci. Manuf. 2017, 92, 10–16. [Google Scholar] [CrossRef]
- Feng, Q.; Wu, D.; Zhao, Y.; Wei, A.; Wei, Q.; Fong, H. Electrospun AOPAN/RC blend nanofiber membrane for efficient removal of heavy metal ions from water. J. Hazard. Mater. 2018, 344, 819–828. [Google Scholar] [CrossRef]
- Celebioglu, A.; Demirci, S.; Uyar, T. Cyclodextrin-grafted electrospun cellulose acetate nanofibers via “Click” reaction for removal of phenanthrene. Appl. Surf. Sci. 2014, 305, 581–588. [Google Scholar] [CrossRef]
- Sarioglu, O.F.; Yasa, O.; Celebioglu, A.; Uyar, T.; Tekinay, T. Efficient ammonium removal from aquatic environments by Acinetobacter calcoaceticus STB1 immobilized on an electrospun cellulose acetate nanofibrous web. Green Chem. 2013, 15, 2566–2572. [Google Scholar] [CrossRef] [Green Version]
- Gebru, K.A.; Das, C. Removal of Pb (II) and Cu (II) ions from wastewater using composite electrospun cellulose acetate/titanium oxide (TiO2) adsorbent. J. Water Process Eng. 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Choi, H.Y.; Bae, J.H.; Hasegawa, Y.; An, S.; Kim, I.S.; Lee, H.; Kim, M. Thiol-functionalized cellulose nanofiber membranes for the effective adsorption of heavy metal ions in water. Carbohydr. Polym. 2020, 234, 115881. [Google Scholar] [CrossRef] [PubMed]
- Mautner, A.; Bismarck, A. Bacterial nanocellulose papers with high porosity for optimized permeance and rejection of nm-sized pollutants. Carbohydr. Polym. 2021, 251, 117130. [Google Scholar] [CrossRef] [PubMed]
- Bardet, R. Nanocelluloses as Potential Materials for Specialty Papers. Ph.D. Dissertation, Université de Grenoble, Grenoble, France, 2014. [Google Scholar]
- Metreveli, G.; Wågberg, L.; Emmoth, E.; Belák, S.; Strømme, M.; Mihranyan, A. A size-exclusion nanocellulose filter paper for virus removal. Adv. Healthc. Mater. 2014, 3, 1546–1550. [Google Scholar] [CrossRef] [PubMed]
- Mautner, A.; Lee, K.Y.; Lahtinen, P.; Hakalahti, M.; Tammelin, T.; Li, K.; Bismarck, A. Nanopapers for organic solvent nanofiltration. Chem. Commun. 2014, 50, 5778–5781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karim, Z.; Svedberg, A.; Lee, K.-Y.; Khan, M.J. Processing-structure-property correlation understanding of microfibrillated cellulose based dimensional structures for ferric ions removal. Sci. Rep. 2019, 9, 10277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mautner, A.; Kobkeatthawin, T.; Mayer, F.; Plessl, C.; Gorgieva, S.; Kokol, V.; Bismarck, A. Rapid water softening with TEMPO-oxidized/phosphorylated nanopapers. Nanomaterials 2019, 9, 136. [Google Scholar] [CrossRef] [Green Version]
- Jämsä, M.; Kosourov, S.; Rissanen, V.; Hakalahti, M.; Pere, J.; Ketoja, J.A.; Tammelin, T.; Allahverdiyeva, Y. Versatile templates from cellulose nanofibrils for photosynthetic microbial biofuel production. J. Mater. Chem. A 2018, 6, 5825–5835. [Google Scholar] [CrossRef] [Green Version]
- Mautner, A.; Mayer, F.; Hervy, M.; Lee, K.-Y.; Bismarck, A. Better together: Synergy in nanocellulose blends. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170043. [Google Scholar] [CrossRef] [Green Version]
- Sheikhi, A. Emerging cellulose-based nanomaterials and nanocomposites. In Nanomaterials and Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 307–351. [Google Scholar]
- Gholami Derami, H.; Jiang, Q.; Ghim, D.; Cao, S.; Chandar, Y.J.; Morrissey, J.J.; Jun, Y.-S.; Singamaneni, S. A robust and scalable polydopamine/bacterial nanocellulose hybrid membrane for efficient wastewater treatment. ACS Appl. Nano Mater. 2019, 2, 1092–1101. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, Y.; Li, M.; Liu, K.; Hu, C.; Yan, K.; Sun, G.; Wang, D. Activable carboxylic acid functionalized crystalline nanocellulose/PVA-co-PE composite nanofibrous membrane with enhanced adsorption for heavy metal ions. Sep. Purif. Technol. 2017, 186, 70–77. [Google Scholar] [CrossRef]
- Wahid, F.; Zhao, X.-J.; Duan, Y.-X.; Zhao, X.-Q.; Jia, S.-R.; Zhong, C. Designing of bacterial cellulose-based superhydrophilic/underwater superoleophobic membrane for oil/water separation. Carbohydr. Polym. 2021, 257, 117611. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Ghim, D.; Cao, S.; Tadepalli, S.; Liu, K.-K.; Kwon, H.; Luan, J.; Min, Y.; Jun, Y.-S.; Singamaneni, S. Photothermally active reduced graphene oxide/bacterial nanocellulose composites as biofouling-resistant ultrafiltration membranes. Environ. Sci. Technol. 2018, 53, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Zhang, T.; Yang, D.; Qiu, F.; Li, Z. Janus ZnO-cellulose/MnO2 hybrid membranes with asymmetric wettability for highly-efficient emulsion separations. Cellulose 2018, 25, 5951–5965. [Google Scholar] [CrossRef]
- Lotfikatouli, S.; Hadi, P.; Yang, M.; Walker, H.W.; Hsiao, B.S.; Gobler, C.; Reichel, M.; Mao, X. Enhanced anti-fouling performance in Membrane Bioreactors using a novel cellulose nanofiber-coated membrane. Sep. Purif. Technol. 2021, 275, 119145. [Google Scholar] [CrossRef]
- Iglesias, R.; Simón, P.; Moragas, L.; Arce, A.; Rodriguez-Roda, I. Cost comparison of full-scale water reclamation technologies with an emphasis on membrane bioreactors. Water Sci. Technol. 2017, 75, 2562–2570. [Google Scholar] [CrossRef]
- Judd, S.J. Membrane technology costs and me. Water Res. 2017, 122, 1–9. [Google Scholar] [CrossRef]
- Bhutiya, P.L.; Mahajan, M.S.; Rasheed, M.A.; Pandey, M.; Hasan, S.Z.; Misra, N. Zinc oxide nanorod clusters deposited seaweed cellulose sheet for antimicrobial activity. Int. J. Biol. Macromol. 2018, 112, 1264–1271. [Google Scholar] [CrossRef]
- Nelson, K.; Retsina, T.; Iakovlev, M.; van Heiningen, A.; Deng, Y.; Shatkin, J.A.; Mulyadi, A. American process: Production of low cost nanocellulose for renewable, advanced materials applications. In Materials Research for Manufacturing; Springer: Berlin/Heidelberg, Germany, 2016; pp. 267–302. [Google Scholar]












| Properties | CNFs | CNCs | BNCs |
|---|---|---|---|
| Diameter (nm) | <100 | 1–100 | 20–100 |
| Length (nm) | Micrometer range | 5–200 | Micrometer range |
| Morphology | Long Chain | Rod/needle | Twisted ribbon |
| Tensile Modulus (GPa) | 100 | 130 | 80–110 |
| Tensile Strength (GPa) | 0.8–1.0 | 8–10 | 1.5–1.7 |
| Crystallinity (%) | 50–65 | 72–80 | 75–80 |
| Aspect Ratio | 60–100 | 10–50 | ~50 |
| Specific Surface Area (m2/g) | 51 | 533 | 125 |
| Source of Nanocellulose | Reference | |
|---|---|---|
| Wood Feedstock | Hemlock | [22] |
| fir, poplar, beech cherry wood | [23] | |
| spruce | [24] | |
| white cedar | [25] | |
| pine, aspen | [26] | |
| Armand pine | [27] | |
| American elm, red maple, paper birch | [28] | |
| eucalyptus wood | [29] | |
| oak | [30] | |
| Agricultural Residues and Plants | coconut shell, rice husk | [31] |
| Miscanthus | [32] | |
| rice Straw | [33] | |
| hazelnut shell | [24] | |
| switch grass | [34] | |
| Napier grass, jute fiber, Bermuda grass, coffee pulp | [35] | |
| elephant grass | [36] | |
| orchard grass, esparto grass and timothy grass | [37] | |
| oat straw | [38] | |
| sugarcane bagasse | [21] | |
| corn cobs, wheat straw, bamboo | [25] | |
| sisal hemp | [39] | |
| banana | [40] | |
| soybean hulls | [41] | |
| soybean straw | [42] | |
| barley straw, sweet sorghum bagasse | [43] | |
| cotton stalk | [44] | |
| pineapple leaf, sunflower stalk | [36] | |
| water hyacinth | [45] | |
| Algae | algae | [46] |
| bacteria | bacteria | [47] |
| Waste | municipal solid waste | [48] |
| Adsorbent Type | Targeted Dye | Production Method | Optimum Condition | Maximum Adsorption mg/g | Reusability | Reference |
|---|---|---|---|---|---|---|
| Carboxylated CNC | Methylene blue | Tempo oxidation | pH 9.0 | 769 | - | [139] |
| Carboxylated CNCs | Methylene blue | Citric acid-hydrochloric acid hydrolysis | - | 92.80% | - | [140] |
| Lignocellulosic Materials | Methylene blue | Neem oil-phenolic resin processed lignocellulosic materials | pH 2–8, time 5 min | 2000 | five cycles | [141] |
| Cellulose Nanofibers (CNFs) | Crystal violet dyes | Nonsolvent- supported approach by applying Meldrum’s acid as an esterification agent | - | 3.984 | - | [142] |
| dxe | Methylene blue | Triple-layered thin film composite nanofiltration membrane | - | 96.70% | - | [143] |
| Microfribillated cellulose dialdehyde—chitosan composite | Congo red | Microcrystalline cellulose with high-pressure homogenization | Time 10 min | 152.5 | - | [144] |
| TEMPO-oxidized cellulose nanofibers/TiO2 nanocomposite | Brilliant Blue | TEMPO-oxidation accompanied by precipitation | Temp 25 °C, pH 3–8, Time 5 to 240 min | 162 | - | [145] |
| Sulphated CNCs | Auramine O | One-step ammonium persulphate oxidation | Temp 0 °C and 25 °C, Time 30 min | 20 | - | [146] |
| Electrosterically stabilized nanocryltalline cellulose | Methylene blue | Two-step oxidation by periodate and chlorite | Temp 20–60 °C, pH 1–10, Time 1 h | 1250 | four cycles | [147] |
| CNCs incorporated by Zno Nanoparticle | Methylene blue | One-pot Synthesis | Temp 25–45 °C, pH 2–10, Time 24 h | 64.9 | four cycles | [148] |
| CNC-polydopamine composite | Methylene blue | self-polymerization | Temp 25 °C, pH 2–11, Time 24 h | 2066.7 | four cycles | [149] |
| graphene oxide polymer aerogel/Magnetic BNC | Malachite Green | Combination of simple filler-loaded networks process with the aid of vacuum freeze-drying | Temp 5–45 °C, pH 2–12, Time 5–25 min | 270.2 | eight cycles | [150] |
| CNC modified by Surfactant | Congo red | Sonication | At room temperature, pH 7.5, Time 2 h | 448 | five cycles | [151] |
| Cellulose microcrystalline | Disperse yellow Dye | Surface modification | Temp 25 °C, pH 11, Time 10 min | 30 | - | [152] |
| Sodium periodate-modified nanocellulose prepared from Eichhornia crassipes | Methylene Blue | A complicated chemical process | Temp 25 °C, pH 8.0, Time 1 h | 90.91 | Thirteen cycles | [153] |
| Ethylenediamine tetra-acetic acid embedded nanocellulose | Methylene Blue | Embedment method | Temp 30 °C, pH 10 | 98% | - | [154] |
| Nanocellulose for immobilization of Laccase | malachite green and congo red | enzyme immobilization | Temp 50 °C, pH 5 for malachite green; pH 6 for congo red, Time 1 h | 92% for malachite green and 62% for congo red | Eighteen cycles | [155] |
| Separator Type | Production Method | Water Contact Angle | Separation Efficiency | Reference |
|---|---|---|---|---|
| cellulose/poly (vinyl alcohol) composite aerogels | chemical cross-linking, freeze drying, and silanization | 156.6° | 98.5% | [171] |
| Holocellulose nanofibers | TEMPO-Mediated oxidation | 149° | 98.5% | [172] |
| Cellulose nanocrystals/polyvinyl alcohol/tetraethyl orthosilicate aerogel | Freeze-drying method | 154.93° ± 4.14° | 92% | [173] |
| Modification of wood and cotton fabric through Octadecylamine | Grafting | 168.2° | 97% | [174] |
| CNF- polydimethylsiloxane | Freez drying method | 163.5° | 99.9% | [175] |
| Cellulose Based Nanopapers | Production Method | Targeted Materials | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|
| Cellulose Nanofibers | freeze-dried | Iron | 53 | [200] |
| Ethanol phosphorylated TEMPO-oxidized cellulose nanofibrils | Cellulose nanofibrils produced from fibre sludge | Ca (II) & Mg (II) | 90 & 70 respectively | [201] |
| TEMPO-oxidised cellulose nanofibrils | sustainable biofuels manufacturing from green algae and cyanobacteria | Ca (II) | - | [202] |
| Nanopaper prepared by carboxylated CNFs | - | metal ions | - | [203] |
| Nanopaper made from pristine fibrous NC | - | filtration of viruses and nanoparticles | - | [204] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, D.; Zhao, Y.; Zhao, R.; Russell, S.J.; Ning, X. A Review on Nanocellulose and Superhydrophobic Features for Advanced Water Treatment. Polymers 2022, 14, 2343. https://doi.org/10.3390/polym14122343
Iqbal D, Zhao Y, Zhao R, Russell SJ, Ning X. A Review on Nanocellulose and Superhydrophobic Features for Advanced Water Treatment. Polymers. 2022; 14(12):2343. https://doi.org/10.3390/polym14122343
Chicago/Turabian StyleIqbal, Danish, Yintao Zhao, Renhai Zhao, Stephen J. Russell, and Xin Ning. 2022. "A Review on Nanocellulose and Superhydrophobic Features for Advanced Water Treatment" Polymers 14, no. 12: 2343. https://doi.org/10.3390/polym14122343
APA StyleIqbal, D., Zhao, Y., Zhao, R., Russell, S. J., & Ning, X. (2022). A Review on Nanocellulose and Superhydrophobic Features for Advanced Water Treatment. Polymers, 14(12), 2343. https://doi.org/10.3390/polym14122343