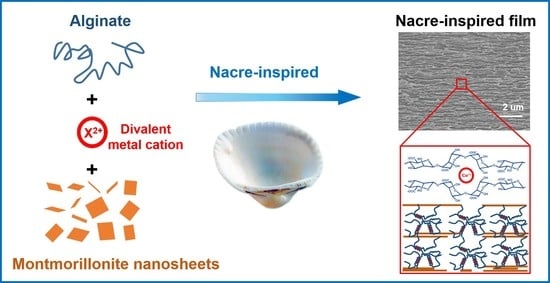
Bioinspired High-Strength Montmorillonite-Alginate Hybrid Film: The Effect of Different Divalent Metal Cation Crosslinking
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of MMT-ALG-X2+ Hybrid Film
2.3. Characterization
3. Results and Discussion
3.1. Microstructure of MMT/ALG-X2+ Nacre-like Film
3.2. Ionic Crosslinking and Interfacial Interaction
3.3. Mechanical Property
3.4. Transparency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Doineau, E.; Cathala, B.; Benezet, J.C.; Bras, J.; Le Moigne, N.; Network, E.P. Development of Bio-Inspired Hierarchical Fibres to Tailor the Fibre/Matrix Interphase in (Bio)composites. Polymers 2021, 13, 804. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; Lim, C.T.; Li, A.; Nizam, B.R.H.; Tan, E.P.S.; Seki, Y.; McKittrick, J. The role of organic intertile layer in abalone nacre. Mater. Sci. Eng. C Mater. Biol. Appl. 2009, 29, 2398–2410. [Google Scholar] [CrossRef]
- Smith, B.L.; Schäffer, T.E.; Viani, M.; Thompson, J.B.; Frederick, N.A.; Kindt, J.; Belcher, A.; Stucky, G.D.; Morse, D.E.; Hansma, P.K. Molecular mechanistic origin of the toughness of natural adhesives, fibres and composites. Nature 1999, 399, 761–763. [Google Scholar] [CrossRef]
- Wang, R.Z.; Suo, Z.; Evans, A.G.; Yao, N.; Aksay, I.A. Deformation mechanisms in nacre. J. Mater. Res. 2001, 16, 2485–2493. [Google Scholar] [CrossRef]
- Huang, W.; Restrepo, D.; Jung, J.Y.; Su, F.Y.; Liu, Z.Q.; Ritchie, R.O.; McKittrick, J.; Zavattieri, P.; Kisailus, D. Multiscale Toughening Mechanisms in Biological Materials and Bioinspired Designs. Adv. Mater. 2019, 31, 1901561. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, L. Nacre-Inspired Structural Composites: Performance-Enhancement Strategy and Perspective. Adv. Mater. 2018, 29, 1702903. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, P.; Zhou, J.; Qi, S.; Yamauchi, Y.; Shi, R.; Fang, R.; Ishida, Y.; Wang, S.; Tomsia, A.P.; et al. Layered nanocomposites by shear-flow-induced alignment of nanosheets. Nature 2020, 580, 210–215. [Google Scholar] [CrossRef]
- Zhao, X.M.; Li, W.; Wang, Y.J.; Li, H.; Wang, J.F. Bioinspired modified graphite film with superb mechanical and thermoconductive properties. Carbon 2021, 181, 40–47. [Google Scholar] [CrossRef]
- Naveen, J.; Jawaid, M.; Goh, K.L.; Reddy, D.M.; Muthukumar, C.; Loganathan, T.M.; Reshwanth, K.N.G.L. Advancement in Graphene-Based Materials and Their Nacre Inspired Composites for Armour Applications—A Review. Nanomaterials 2021, 11, 1239. [Google Scholar] [CrossRef]
- Machado, I.; Hsieh, I.; Calado, V.; Chapin, T.; Ishida, H. Nacre-Mimetic Green Flame Retardant: Ultra-High Nanofiller Content, Thin Nanocomposite as an Effective Flame Retardant. Polymers 2020, 12, 2351. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Cheng, Q. Role of Interface Interactions in the Construction of GO-Based Artificial Nacres. Adv. Mater. Interfaces 2018, 5, 1800107. [Google Scholar] [CrossRef]
- Cheng, Q.F.; Duan, J.L.; Zhang, Q.; Jiang, L. Learning from Nature: Constructing Integrated Graphene-Based Artificial Nacre. ACS Nano 2015, 9, 2231–2234. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.C.; Sun, W.J.; Zhou, C.G.; Yan, D.X.; Zhang, Q.C.; Li, Z.M. Integrated strength and toughness in graphene/calcium alginate films for highly efficient electromagnetic interference shielding. J. Mater. Chem. C 2018, 6, 9166–9174. [Google Scholar] [CrossRef]
- Du, G.L.; Wu, F.X.; Cong, Y.; Nie, L.; Liu, S.H.; Gao, G.R.; Fu, J. Versatile controlled ion release for synthesis of recoverable hybrid hydrogels with high stretchability and notch-insensitivity. Chem. Commun. 2015, 51, 15534–15537. [Google Scholar] [CrossRef]
- Zhou, Q.; Kang, H.F.; Bielec, M.; Wu, X.P.; Cheng, Q.; Wei, W.Y.; Dai, H.L. Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohydr. Polym. 2018, 197, 292–304. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhao, X.H.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z.G. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- Liang, B.L.; Zhao, H.W.; Zhang, Q.; Fan, Y.Z.; Yue, Y.H.; Yin, P.G.; Guo, L. Ca2+ Enhanced Nacre-Inspired Montmorillonite-Alginate Film with Superior Mechanical, Transparent, Fire Retardancy, and Shape Memory Properties. ACS Appl. Mater. Interfaces 2016, 8, 28816–28823. [Google Scholar] [CrossRef]
- Liang, B.L.; Wang, J.F.; Shu, Y.Q.; Yin, P.G.; Guo, L. A biomimetic ion-crosslinked layered double hydroxide/alginate hybrid film. RSC Adv. 2017, 7, 32601–32606. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.H.; Wang, M.X.; Haider, H.; Yang, J.H.; Sun, J.Y.; Chen, Y.M.; Zhou, J.X.; Suo, Z.G. Strengthening Alginate/Polyacrylamide Hydrogels Using Various Multivalent Cations. ACS Appl. Mater. Interfaces 2013, 5, 10418–10422. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, D.; Alvarez, C.; Mullen, A.M. Biodegradable Packaging Materials from Animal Processing Co-Products and Wastes: An Overview. Polymers 2021, 13, 2561. [Google Scholar] [CrossRef] [PubMed]
- Agulhon, P.; Robitzer, M.; David, L.; Quignard, F. Structural Regime Identification in Ionotropic Alginate Gels: Influence of the Cation Nature and Alginate Structure. Biomacromolecules 2012, 13, 215–220. [Google Scholar] [CrossRef]
- Agulhon, P.; Markova, V.; Robitzer, M.; Quignard, F.; Mineva, T. Structure of Alginate Gels: Interaction of Diuronate Units with Divalent Cations from Density Functional Calculations. Biomacromolecules 2012, 13, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Menakbi, C.; Quignard, F.; Mineva, T. Complexation of Trivalent Metal Cations to Mannuronate Type Alginate Models from a Density Functional Study. J. Phys. Chem. B 2016, 120, 3615–3623. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Wei, H.; Zhang, Q.; Zhang, X.Z.; Cheng, S.X.; Zhuo, R.X. Effect of ions on the aggregation behavior of natural polymer alginate. J. Phys. Chem. B 2009, 113, 14839–14843. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Sarker, B.; Papageorgiou, D.G.; Silva, R.; Zehnder, T.; Gul-E-Noor, F.; Bertmer, M.; Kaschta, J.; Chrissafis, K.; Detsch, R.; Boccaccini, A.R. Fabrication of alginate-gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J. Mater. Chem. B 2014, 2, 1470–1482. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Shi, Z.; Lian, M.; Li, H.; Yin, J. Mechanically strong graphene oxide/sodium alginate/polyacrylamide nanocomposite hydrogel with improved dye adsorption capacity. J. Mater. Chem. A 2013, 1, 7433–7443. [Google Scholar] [CrossRef]
- Shu, Y.Q.; Yin, P.G.; Wang, J.F.; Liang, B.L.; Wang, H.; Guo, L. Bioinspired Nacre-like Heparin/Layered Double Hydroxide Film with Superior Mechanical, Fire-Shielding, and UV-Blocking Properties. Ind. Eng. Chem. Res. 2014, 53, 3820–3826. [Google Scholar] [CrossRef]
- Liang, B.-L.; Shu, Y.-Q.; Yin, P.-G.; Guo, L. Nacre-inspired polyglutamic acid/layered double hydroxide bionanocomposite film with high mechanical, translucence and UV-blocking properties. Chin. J. Polym. Sci. 2017, 35, 631–640. [Google Scholar] [CrossRef]
- Yao, H.-B.; Tan, Z.-H.; Fang, H.-Y.; Yu, S.-H. Artificial Nacre-like Bionanocomposite Films from the Self-Assembly of Chitosan-Montmorillonite Hybrid Building Blocks. Angew. Chem. Int. Ed. 2010, 49, 10127–10131. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Bjurhager, I.; Malho, J.-M.; Pere, J.; Ruokolainen, J.; Berglund, L.A.; Ikkala, O. Large-Area, Lightweight and Thick Biomimetic Composites with Superior Material Properties via Fast, Economic, and Green Pathways. Nano Lett. 2010, 10, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Sehaqui, H.; Kochumalayil, J.; Liu, A.D.; Zimmermann, T.; Berglund, L.A. Multifunctional Nanoclay Hybrids of High Toughness, Thermal, and Barrier Performances. ACS Appl. Mater. Interfaces 2013, 5, 7613–7620. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Q.; Lin, L.; Chen, L.; Jiang, L. Understanding the relationship of performance with nanofiller content in the biomimetic layered nanocomposites. Nanoscale 2013, 5, 6356–6362. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, T.; Du, Y.; Dou, S.; Zhang, H.; Jiang, L.; Cheng, Q. Strong bioinspired HPA-rGO nanocomposite films via interfacial interactions for flexible supercapacitors. Nano Energy 2019, 58, 517–527. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Kaushik, A.K.; Arruda, E.M.; Waas, A.M.; Shim, B.S.; Xu, J.D.; Nandivada, H.; Pumplin, B.G.; Lahann, J.; Ramamoorthy, A.; et al. Ultrastrong and stiff layered polymer nanocomposites. Science 2007, 318, 80–83. [Google Scholar] [CrossRef]
- Ming, P.; Song, Z.F.; Gong, S.S.; Zhang, Y.Y.; Duan, J.L.; Zhang, Q.; Jiang, L.; Cheng, Q.F. Nacre-inspired integrated nanocomposites with fire retardant properties by graphene oxide and montmorillonite. J. Mater. Chem. A 2015, 3, 21194–21200. [Google Scholar] [CrossRef]
- Shu, Y.Q.; Yin, P.G.; Liang, B.L.; Wang, H.; Guo, L. Bioinspired Design and Assembly of Layered Double Hydroxide/Poly(vinyl alcohol) Film with High Mechanical Performance. ACS Appl. Mater. Interfaces 2014, 6, 15154–15161. [Google Scholar] [CrossRef]
- Barthelat, F.; Li, C.M.; Comi, C.; Espinosa, H.D. Mechanical properties of nacre constituents and their impact on mechanical performance. J. Mater. Res. 2006, 21, 1977–1986. [Google Scholar] [CrossRef] [Green Version]
- Faber, K.T.; Evans, A.G. Crack deflection processes—I. Theory. Acta Metall. 1983, 31, 565–576. [Google Scholar] [CrossRef]
- Wang, R.Z.; Wen, H.B.; Cui, F.Z.; Zhang, H.B.; Li, H.D. Observations of damage morphologies in nacre during deformation and fracture. J. Mater. Sci. 1995, 30, 2299–2304. [Google Scholar] [CrossRef]
- Wan, S.; Cheng, Q. Fatigue-Resistant Bioinspired Graphene-Based Nanocomposites. Adv. Funct. Mater. 2017, 27, 1703459. [Google Scholar] [CrossRef]
- Ni, H.; Liu, X.; Cheng, Q. A new strategy for air-stable black phosphorus reinforced PVA nanocomposites. J. Mater. Chem. A 2018, 6, 7142–7147. [Google Scholar] [CrossRef]
- Bonderer, L.J.; Studart, A.R.; Woltersdorf, J.; Pippel, E.; Gauckler, L.J. Strong and ductile platelet-reinforced polymer films inspired by nature: Microstructure and mechanical properties. J. Mater. Res. 2009, 24, 2741–2754. [Google Scholar] [CrossRef] [Green Version]
- Bonderer, L.J.; Studart, A.R.; Gauckler, L.J. Bioinspired design and assembly of platelet reinforced polymer films. Science 2008, 319, 1069–1073. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, H.; Cheng, Q. Multiple Synergistic Toughening Graphene Nanocomposites through Cadmium Ions and Cellulose Nanocrystals. Adv. Mater. Interfaces 2018, 5, 1800145. [Google Scholar] [CrossRef]
- Cong, H.P.; Wang, P.; Yu, S.H. Highly Elastic and Superstretchable Graphene Oxide/Polyacrylamide Hydrogels. Small 2014, 10, 448–453. [Google Scholar] [CrossRef]
- Wan, S.J.; Hu, H.; Peng, J.S.; Li, Y.C.; Fan, Y.Z.; Jiang, L.; Cheng, Q.F. Nacre-inspired integrated strong and tough reduced graphene oxide-poly(acrylic acid) nanocomposites. Nanoscale 2016, 8, 5649–5656. [Google Scholar] [CrossRef]
- Compton, O.C.; Cranford, S.W.; Putz, K.W.; An, Z.; Brinson, L.C.; Buehler, M.J.; Nguyen, S.T. Tuning the Mechanical Properties of Graphene Oxide Paper and Its Associated Polymer Nanocomposites by Controlling Cooperative Intersheet Hydrogen Bonding. ACS Nano 2012, 6, 2008–2019. [Google Scholar] [CrossRef]
- Putz, K.W.; Compton, O.C.; Palmeri, M.J.; Nguyen, S.T.; Brinson, L.C. High-Nanofiller-Content Graphene Oxide-Polymer Nanocomposites via Vacuum-Assisted Self-Assembly. Adv. Funct. Mater. 2010, 20, 3322–3329. [Google Scholar] [CrossRef]
- Medhekar, N.V.; Ramasubramaniam, A.; Ruoff, R.S.; Shenoy, V.B. Hydrogen Bond Networks in Graphene Oxide Composite Paper: Structure and Mechanical Properties. ACS Nano 2010, 4, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, Q.; Zhu, S.P. Alginate Hydrogel: A Shapeable and Versatile Platform for in Situ Preparation of Metal-Organic Framework-Polymer Composites. ACS Appl. Mater. Interfaces 2016, 8, 17395–17401. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-F.; Gao, H.-L.; Lu, Y.; Wu, C.-Y.; Wu, Y.-D.; Wang, X.-Y.; Pan, Z.-Q.; Dong, L.; Song, Y.-H.; Cong, H.-P.; et al. Transforming ground mica into high-performance biomimetic polymeric mica film. Nat. Commun. 2018, 9, 2974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, P.; Malho, J.-M.; Rahimi, K.; Schacher, F.H.; Wang, B.; Demco, D.E.; Walther, A. Nacre-mimetics with synthetic nanoclays up to ultrahigh aspect ratios. Nat. Commun. 2015, 6, 5967. [Google Scholar] [CrossRef] [Green Version]
- Mu, Z.C.; Wu, S.F.; Huang, X.B.; Zhang, W.; Yi, J.Y.; Jiang, N.J. High Elongation and Transparent Nacre-Inspired PVA/MMT Nanocomposites. Macromol. Rapid Commun. 2021, 42, 2100229. [Google Scholar] [CrossRef]
- Yao, H.-B.; Fang, H.-Y.; Tan, Z.-H.; Wu, L.-H.; Yu, S.-H. Biologically Inspired, Strong, Transparent, and Functional Layered Organic-Inorganic Hybrid Films. Angew. Chem. Int. Ed. 2010, 49, 2140–2145. [Google Scholar] [CrossRef]
- Aulin, C.; Salazar-Alvarez, G.; Lindstrom, T. High strength, flexible and transparent nanofibrillated cellulose-nanoclay biohybrid films with tunable oxygen and water vapor permeability. Nanoscale 2012, 4, 6622–6628. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Song, T.; Chen, H.; Ming, W.; Cheng, Z.; Liu, J.; Liang, B.; Wang, Y.; Wang, G. Bioinspired High-Strength Montmorillonite-Alginate Hybrid Film: The Effect of Different Divalent Metal Cation Crosslinking. Polymers 2022, 14, 2433. https://doi.org/10.3390/polym14122433
Wang J, Song T, Chen H, Ming W, Cheng Z, Liu J, Liang B, Wang Y, Wang G. Bioinspired High-Strength Montmorillonite-Alginate Hybrid Film: The Effect of Different Divalent Metal Cation Crosslinking. Polymers. 2022; 14(12):2433. https://doi.org/10.3390/polym14122433
Chicago/Turabian StyleWang, Jiaen, Tianliang Song, Huaxiang Chen, Wei Ming, Zhiming Cheng, Jingwen Liu, Benliang Liang, Yuting Wang, and Guangsheng Wang. 2022. "Bioinspired High-Strength Montmorillonite-Alginate Hybrid Film: The Effect of Different Divalent Metal Cation Crosslinking" Polymers 14, no. 12: 2433. https://doi.org/10.3390/polym14122433
APA StyleWang, J., Song, T., Chen, H., Ming, W., Cheng, Z., Liu, J., Liang, B., Wang, Y., & Wang, G. (2022). Bioinspired High-Strength Montmorillonite-Alginate Hybrid Film: The Effect of Different Divalent Metal Cation Crosslinking. Polymers, 14(12), 2433. https://doi.org/10.3390/polym14122433