Advanced Polymeric Nanocomposites for Water Treatment Applications: A Holistic Perspective
Abstract
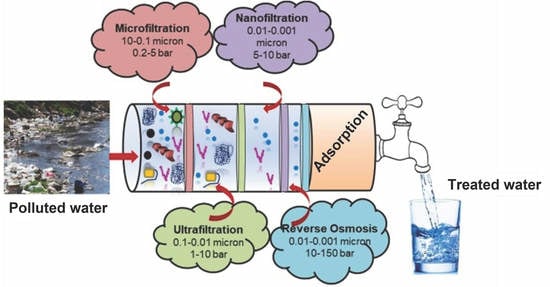
:1. Introduction
2. Synthetic Methods and Remedial Application of Polymeric Nanocomposites
2.1. Dendritic Polymers
Environmental Remediation Using Dendritic Polymers
- They are a unique sort of macromolecules with a highly branched structure, high porosity, and a three-dimensional functionalized structure.
- Dendrimers can be grafted onto large supports, leading to increased selectivity through size exclusion by modified cavities and/or via selective binding to contaminants due to well-tailored support/substrate. Large support may also enhance surface area of the nanocomposite, leading to higher adsorption/separation capacity.
- They have large external and interior regions, as well as a large network of peripheral functional moieties, which permits the capture of large amount of contaminants.
- Adjustment of the physicochemical parameters of the core, interior cells, and outer end groups plays a major role in their adsorption capacity.
- The existence of a high number of required peripheral functional groups ensures good selectivity. More intriguingly, the character of functional groupings of the nanocomposite can be tailored to target pollutants.
| Dendritic Nanocomposites | Target Pollutant | Remediation Approach | Removal Capacity | References |
|---|---|---|---|---|
| PAMAM/Graphene oxide | Heavy metals: Pb, Cd, Cu, MnCd, Cu, Mn | Adsorption | 568.18, 253.81, 68.68, 18.29 253.81, 68.68, 18.29 (mg/g) | [52,53] |
| Dendrimer-clay nanocomposite | Cr | Adsorption | 6–10 (mg/g) | [54] |
| Polystyrene PAMAMiminodiacetic acid | Ni | Adsorption | 24.09 (mg/g) | [55] |
| PAMAM-grafted cellulosenanofibril | Cr | Adsorption | 377.36 (mg/g) | [56] |
| Hyperbranched PAMAM/polysulfone membrane | Cd | Ultrafiltration | 27.29 µg/cm2 | [57] |
| Dendrimer/titania | Pb | Adsorption | 400 (mg/g) | [58] |
| PAMAM-grafted core-shellmagnetic silica nanoparticles | Hg | Adsorption | 134.6 (mg/g) | [59] |
| PAMAM dendrimers withethylenediamine (EDA) core | Cu | Ultrafiltration | 451 (mg/g) | [60] |
| Amine terminated-Magneticcored dendrimer | Pb, Cd | Adsorption | 170.42, 75.15 (mg/g) | [61] |
| Carbon nanotube-dendrimer | Pb, Cu | Adsorption | 3333–4320 (mg/g) | [62] |
| Polyacrylonitrile/PAMAM composite nanofibers, | Dyes: Direct red 80, Direct red 23 | Adsorption | 2000 (mg/g) | [63] |
| Magnetic Chitosan/PAMAM | Reactive blue 21 | Adsorption | 555.56 (mg/g) | [32] |
| PPI–grafted cotton fabrics | Direct red 80, Disperse yellow 42, Basic blue 9 | Adsorption | 143.3, 104.8, 105.8 (mg/g) | [64] |
| PPI dendrimer | Direct red 80, Acid green 25, Acid blue 7, Direct red 23 | Adsorption | 33,333–50,000 (mg/g) | [49] |
| Graphene oxide-PPI dendrimer | Acid red 14, Acid blue 92 | Adsorption | 434.78, 196.08 (mg/g) | [65] |
| PAMAM–titaniananohybrid | Phenol | Adsorption | 77 (mg/g) | [61] |
| PPI dendrimers functionalized with long aliphatic chains | PAHs: Fluoranthene, Phenanthrene, Pyrene | Adsorption | 19, 67, 57 (mg/g) | [66] |
| Alkylated hyperbranched polymers | Fluoranthene, Phenanthrene, Pyrene | Adsorption | 6–54 (mg/g) | [45] |
2.2. Polymeric Aerogels and Hydrogels
| Material Description | Core Findings | Reference |
|---|---|---|
| MnO2 coated cellulose nanofibers | Oxidation occurred at acidic pH. Over 99.8% removal of methylene blue dye | [83] |
| MnO2/graphene aerogel (GMA) | GMA had 100% adsorption of rhodamine B and 89.02% COD, compared to 73.80% and 59.65% for SMA (silica wool-MnO2 deposition) | [81] |
| Poly(acrylic acid)/starch hydrogel | Adsorption of cadmium was best described by Langmuir (monolayer) adsorption model with a maximum adsorption capacity of 588 mg/g | [84] |
| 3D MnO2 modified biochar-based porous hydrogels | Cd(II) and Pb(II) removal from aquatic and soil systems could be possible uses. Reusable and highly stable | [85] |
| Cassava starch-based double network hydrogel | The high adsorption capacity of about 417 mg/g and adsorption performance of 70% after regeneration five times. Physically and mechanically stable. | [86] |
| Chitosan-Gelatin based hydrogel | CH-GEL/ZSPNC (MW) eliminated 99% of cationic dye from the solution. The adsorption capacity of about 10.5 mg/g | [87] |
| CdS amended nano-ZnO/chitosan hydrogel | For 5.0 mg/L, 95 percent of Congo Red was removed in 1 min. Pollutant removal is quick, with high apparent rate constants and good reusability. | [88] |
| MnO2 NWs/chitosan hydrogels | Abundant sunlight absorption (94%). The conversion efficiency of sunlight to thermal energy (90.6%) | [89] |
2.3. Polymeric Membrane and Biopolymers
| Polymeric Membrane | Treatment Technology | Target Pollutants | Core Process Conditions | Reference |
|---|---|---|---|---|
| ES-10- polyamide, NTR-729HF- polyvinyl alcohol | Reverse osmosis (RO) | As, Sb | As(V) and Sb(V) removals are substantially higher than As(III) and Sb(V) removals at pH 3–10. | [108] |
| ES-10 and HS5110/HR3155 | Nanofiltration (NF)/RO | As | NF: pressure 0.2–0.7 MPa/RO: pressure 4 MPa | [109] |
| NF90–4040 | NF | Cr, As | pH = 9, temp. 45 °C, pressure 3.1 MPa | [110] |
| UiO-66 (Zr-MOF)/TFN | NF | Se, As | 1.15 L/m2·h/MPa | [111] |
| The P[MPC-co-AEMA] co-polymer | NF | Se, As | 0.85 L/m2·h/MPa | [112] |
| PVDF with melanin nanoparticles from the marine bacterium Pseudomonas stutzeri | Vacuum filtration (VF) | Hg, Cu, Cr, Pb | 45 °C; pH = 3 for Cr and pH = 5 for other metals; flow rate of 0.5 mL/min | [113] |
| M-I | Micellar enhanced filtration (MEF) | Cu, Pb, Cd | Operating pressure 0.025 MPa; the flux 63,579 L/m2 h | [114] |
| PAN- Polyacrylonitrile—Osmonic 100 kDa | Electro-ultrafiltration (EUF) | As | an averaged crossflow velocity of 0.1 m/s; pressure 0.098 MPa | [115] |
| Desal AG-2540 RO, TFC-ULP-2540 RO, and TFC-SR2-2540 NF | NF/RO | Sr | Applied pressure 0.10–0.15 MPa, pH = 3–6 | [116] |
| Polyelectrolyte multilayer membrane | NF | Mg, Sr, Ca, Ba | low ionic strength conditions (e.g., <50 mM NaCl as a background electrolyte); 0.345 MPa; crossflow velocity 21.4 cm/s; 25 °C. | [117] |
| tubular Kerasep® ceramic membranę | Hybrid: Oxidation | Fe | Oxidation: 0.07 MPa; 20–22 °C; MF: tangential velocity 3.2 m/s; trans-membrane pressure 0.06–0.3 MPa; pH = 6.8–7.2; 20–22 °C | [118] |
| PPSU—sulfonated polyphenylenesulfone polymer; TBF—triangle-shape tri-bore hollow fiber membranes | UF | Oil | Transmembrane pressure of 0.1 MPa; a flow rate of 300 mL/min along the lumen side; a velocity range of 2.58–2.81 m/s | [119] |
| NiCo-LDH—nickel cobalt layered double hydroxide; PVDF—the polydopamine modified polyvinylidenefluoride membrane | Gravity-driven filtration | Soybean oil, petroleum ether, 1,2-dichloroethane, n-hexadecane | Glass sand core filter device; water-in-oil emulsions—the volume ratio of 1:99 | [120] |
| APTES—3-aminopropyltriethoxysilane; ATPR—atomic transfer radical polymerization/Graphene oxide | Filtration | Oil | Polymerization with ATRP; a volume ratio of organics and water: 1:99; the pressure of 0.05 MPa; complex environments, such as 2 M HCl, 2 M NaOH and saturated NaCl; permeation flux 10,000 ± 440 L/m2·h·MPa | [121] |
| Nanofibrous PVDF membrane | Gravity-driven filtration | Oil | Permeability 88 1660 ± 6520 L/m2·h·MPa; water-in-oil emulsions (chloroform, toluene, dichloromethane, and high viscosity oils: D4 and D5) | [122] |
| TiO2-Nanoparticles/PVDF—polydopamine modified polyvinylidenefluoride membrane/TrFE—trifluoro ethylene | Photoreactor | Oily industrial wastewater | The flow rate 100.8 L/h; pH = 4–5.5 | [123] |
| SiO2-NPs/PVDF | Separation | Oil | The pressure of 0.09 MPa; fluxes of over 10,000 L/m2 h | [124] |
| PVDF—polydopamine modified polyvinylidenefluoride membrane | RO | Oil | The cross-flow velocity 2 m/s; operating pressure 6 MPa; crossflow membrane sequencing batch reactor inoculated with isolated tropical halophilic microorganisms | [125] |
| Chitosan–SiO2–glutaraldehyde composite/PVDF- polydopamine modified polyvinylidenefluoride membrane | VDF system | Oil | Separation area ~1.6 cm2; the pressure 0.03 MPa. | [126] |
| TiO2-NP/polydopamine modified polyvinylidenefluoride membrane | Separation | Petroleum ether; n-hexadecane; 1,3,5-trimethylbenzene; diesel oil | Pressure difference of 0.09 MPa; separation area 1.77 cm2; permeation flux for SDS/oil/H2O emulsion: 428 L/m2∙h, 605 L/m2∙h, 524 L/m2∙h, 382 L/m2∙h respectively | [127] |
3. Considerations for Future Research
- (1)
- Fouling has long been a severe issue encountered during polymeric membrane applications in water treatment. Antifouling nanoparticles and surface functionalization are some of the ways to address this challenge (post or pre-treatment) [132]. Future studies should concentrate on inhibiting the growth of microbial colonies on the surface of the membrane, as well as minimizing filler leaching.
- (2)
- In real-world applications, polymer nanocomposites’ availability, reusability, cost, stability, agglomeration, and reactivity are all major concerns. As a result, developing novel, inexpensive, and effective nanofillers and polymeric nanocomposites for adsorptive membrane technology still requires attention.
- (3)
- It is difficult to ensure that the adsorptive material combined with the polymeric membrane is safe and harmless. Some composite materials are hazardous because their application in water purification generates secondary pollution. Environmental health and human safety can be achieved by carrying out comprehensive post-treatment evaluation to determine the quality of the water, its suitability for human consumption, and/or its safety for release into the water bodies.
- (4)
- The development of new materials for polymeric nanocomposites remains a major issue, as most materials have been limited to laboratory-scale testing and advanced field trials are needed. Because many innovative materials are not marketable yet due to high pricing or time-consuming synthesis procedures. There is a need for continuous material science research for sustainable and cost-effective polymeric membranes.
- (5)
- In addition to identifying the necessary steps for scaling up new membranes for large-scale industrial applications, there is a need for the development of facile synthesis methods capable of producing defect-free polymeric membranes, without compromising water treatment efficiency.
- (6)
- Furthermore, models for the prediction of the lifespan of the polymeric membrane, regenerability, and reusability are required. To forecast membrane performance and economic viability, models that take into account the morphology and specific characteristics of the polymeric nanocomposite must be developed and validated.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bushra, R.; Shahadat, M.; Ahmad, A.; Nabi, S.A.; Umar, K.; Oves, M. Synthesis, characterization, antimicrobial activity and applications of polyanilineTi(IV)arsenophosphate adsorbent for the analysis of organic and inorganic pollutants. J. Hazard. Mater. 2014, 264, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Bushra, R. 11—Nanoadsorbents-based polymer nanocomposite for environmental remediation. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 243–260. [Google Scholar]
- UNESCO. The United Nations World Water Development Report 2021: Valuing Water. UNESCO World Water Assessment Programme. Available online: https://unesdocunescoorg/ark:/48223/pf00003757242021 (accessed on 3 May 2022).
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.K.; Singhania, R.R.; Albarico, F.P.J.B.; Pandey, A.; Chen, C.-W.; Dong, C.-D. Organic wastes bioremediation and its changing prospects. Sci. Total Environ. 2022, 824, 153889. [Google Scholar] [CrossRef] [PubMed]
- Adeola, A.O.; Akingboye, A.S.; Ore, O.T.; Oluwajana, O.A. Adewole, A.H.; Olawade, D.B. Crude oil exploration in Africa: So-cio-economic implications, environmental impacts, and mitigation strategies. Environ. Syst. Decis. 2021, 42, 26–50. [Google Scholar] [CrossRef] [PubMed]
- Adeola, A.O.; Ore, O.T.; Fapohunda, O.; Adewole, A.H.; Akerele, D.D.; Akingboye, A.S.; Oloye, F.F. Psychotropic Drugs of Emerging Concerns in Aquatic Systems: Ecotoxicology and Remediation Approaches. Chem. Afr. 2022, 5, 481–508. [Google Scholar] [CrossRef]
- Selwe, K.P.; Thorn, J.P.R.; Desrousseaux, A.O.S.; Dessent, C.E.H.; Sallach, J.B. Emerging contaminant exposure to aquatic systems in the Southern African Development Community. Environ. Toxicol. Chem. 2022, 41, 382–395. [Google Scholar] [CrossRef]
- Hannah, L. Chapter 21—Mitigation: Reducing Greenhouse Gas Emissions, Sinks, and Solutions. In Climate Change Biology, 3rd ed.; Hannah, L., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 439–472. [Google Scholar]
- Adeola, A.; Forbes, P.B.C. Advances in water treatment technologies for removal of polycyclic aromatic hydrocarbons: Existing concepts, emerging trends, and future prospects. Water Environ. Res. 2020, 93, 343–359. [Google Scholar] [CrossRef]
- Singh, V.V. Chapter 2—Green nanotechnology for environmental remediation. In Sustainable Nanotechnology for Environmental Remediation; Koduru, J.R., Karri, R.R., Mubarak, N.M., Bandala, E.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 31–61. [Google Scholar]
- UNEP—United Nations Environment Programme. Global Chemicals Outlook II: From Legacies to Innovative Solutions—Implementing the 2030 Agenda for Sustainable Development; The Global Chemicals Outlook; UNEP: Geneva, Switzerland, 2019. [Google Scholar]
- Sanganyado, E. Policies and regulations for the emerging pollutants in freshwater ecosystems: Challenges and opportunities. Emerg. Freshw. Pollut. 2022, 2022, 361–372. [Google Scholar] [CrossRef]
- Ambika, S.P.P. 10—Environmental remediation by nanoadsorbents-based polymer nanocomposite. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 223–241. [Google Scholar]
- Maurya, A.K.; Gogoi, R.; Manik, G. Polymer-Based Nanocomposites for Removal of Pollutants from Different Environment Using Catalytic Degradation. In Advances in Nanocomposite Materials for Environmental and Energy Harvesting Applications; Shalan, A.E., Hamdy, M.A.S., Lanceros-Méndez, S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 331–368. [Google Scholar]
- Darwish, M.S.A.; Mostafa, M.H.; Al-Harbi, L.M. Polymeric Nanocomposites for Environmental and Industrial Applications. Int. J. Mol. Sci. 2022, 23, 1023. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Sun, Z.; Huang, P.; Li, Y.-Q.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Akpan, E.I.; Shen, X.; Wetzel, B.; Friedrich, K. 2—Design and Synthesis of Polymer Nanocomposites. In Polymer Composites with Functionalized Nanoparticles; Pielichowski, K., Majka, T.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–83. [Google Scholar]
- Hmtshirazi, R.; Mohammadi, T.; Asadi, A.A.; Tofighy, M.A. Electrospun nanofiber affinity membranes for water treatment applications: A review. J. Water Process Eng. 2022, 47, 102795. [Google Scholar] [CrossRef]
- Dhillon, S.K.; Kundu, P.P. Polyaniline interweaved iron embedded in urea–formaldehyde resin-based carbon as a cost-effective catalyst for power generation in microbial fuel cell. Chem. Eng. J. 2021, 431, 133341. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Pardo, S.; Bucio, E. Interaction between Filler and Polymeric Matrix in Nanocomposites: Magnetic Approach and Applications. Polymers 2021, 13, 2998. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jambhulkar, S.; Zhu, Y.; Ravichandran, D.; Kakarla, M.; Vernon, B.; Lott, D.G.; Cornella, J.L.; Shefi, O.; Miquelard-Garnier, G.; et al. 3D printing for polymer/particle-based processing: A review. Compos. B Eng. 2021, 223, 109102. [Google Scholar] [CrossRef]
- Ucankus, G.; Ercan, M.; Uzunoglu, D.; Culha, M. 1—Methods for preparation of nanocomposites in environmental remediation. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–28. [Google Scholar]
- Boikanyo, D.; Masheane, M.L.; Nthunya, L.N.; Mishra, S.B.; Mhlanga, S.D. 5—Carbon-supported photocatalysts for organic dye photodegradation. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 99–138. [Google Scholar]
- Kamal, A.; Ashmawy, M.S.S.; Algazzar, A.M.; Elsheikh, A.H. Fabrication techniques of polymeric nanocomposites: A comprehensive review. Proc. Inst. Mech. Eng. C—J. Mech. Eng. Sci. 2021, 236, 009544062211055662. [Google Scholar]
- Oladipo, A.A. 14—Microwave-assisted synthesis of high-performance polymer-based nanoadsorbents for pollution control. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 337–359. [Google Scholar]
- Bizzarri, B.M.; Fanelli, A.; Botta, L.; Zippilli, C.; Cesarini, S.; Saladino, R. Dendrimeric Structures in the Synthesis of Fine Chemicals. Materials 2021, 14, 5318. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Mou, Q.; Wang, D.; Zhu, X.; Yan, D. Dendritic Polymers for Theranostics. Theranostics 2016, 6, 930–947. [Google Scholar] [CrossRef]
- Sajid, M.; Nazal, M.K.; Ihsanullah; Baig, N.; Osman, A.M. Removal of heavy metals and organic pollutants from water using dendritic polymers based adsorbents: A critical review. Sep. Purif. Technol. 2018, 191, 400–423. [Google Scholar] [CrossRef]
- Sadjadi, S. 13—Dendritic polymers for environmental remediation. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 279–335. [Google Scholar]
- Lyu, Z.; Ding, L.; Huang, A.-T.; Kao, C.-L.; Peng, L. Poly(amidoamine) dendrimers: Covalent and supramolecular synthesis. Mater. Today Chem. 2019, 13, 34–48. [Google Scholar] [CrossRef]
- Wang, P.; Ma, Q.; Hu, D.; Wang, L. Removal of Reactive Blue 21 onto magnetic chitosan microparticles functionalized with polyamidoamine dendrimers. React. Funct. Polym. 2015, 91–92, 43–50. [Google Scholar] [CrossRef]
- Karatas, O.; Keyikoglu, R.; Gengec, N.A.; Vatanpour, V.; Khataee, A. A review on dendrimers in preparation and modification of membranes: Progress, applications, and challenges. Mater. Today Chem. 2021, 23, 100683. [Google Scholar] [CrossRef]
- Eghbali, P.; Gürbüz, M.U.; Ertürk, A.S.; Metin, Ö. In situ synthesis of dendrimer-encapsulated palladium(0) nanoparticles as catalysts for hydrogen production from the methanolysis of ammonia borane. Int. J. Hydrog. Energy 2020, 45, 26274–26285. [Google Scholar] [CrossRef]
- Walter, M.V.; Malkoch, M. Simplifying the synthesis of dendrimers: Accelerated approaches. Chem. Soc. Rev. 2012, 41, 4593–4609. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, R.S.; Choudhary, M.; Kandasubramanian, B. Recent advances in dendrimer-based nanoplatform for cancer treatment: A review. Eur. Polym. J. 2020, 126, 109546. [Google Scholar] [CrossRef]
- Viltres, H.; López, Y.C.; Leyva, C.; Gupta, N.K.; Naranjo, A.G.; Acevedo–Peña, P. Polyamidoamine dendrimer-based materials for environmental applications: A review. J. Mol. Liq. 2021, 334, 116017. [Google Scholar] [CrossRef]
- Arkas, M.; Anastopoulos, I.; Giannakoudakis, D.A.; Pashalidis, I.; Katsika, T.; Nikoli, E. Catalytic Neutralization of Water Pol-lutants Mediated by Dendritic Polymers. Nanomaterials 2022, 12, 445. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, K.; Rangasamy, R. Synthetic modification of silica coated magnetite cored PAMAM dendrimer to enrich branched Amine groups and peripheral carboxyl groups for environmental remediation. J. Mol. Struct. 2020, 1224, 129081. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, S.; Xi, C.; Zhang, F. A Review: Adsorption and Removal of Heavy Metals Based on Polyamide-amines Composites. Front. Chem. 2022, 10, 814643. [Google Scholar] [CrossRef]
- Niu, Y.; Qu, R.; Chen, H.; Mu, L.; Liu, X.; Wang, T. Synthesis of silica gel supported salicylaldehyde modified PAMAM dendrimers for the effective removal of Hg(II) from aqueous solution. J. Hazard. Mater. 2014, 278, 267–278. [Google Scholar] [CrossRef]
- Lin, Z.; Pan, Z.; Zhao, Y.; Qian, L.; Shen, J.; Xia, K. Removal of Hg2+ with Polypyrrole-Functionalized Fe3O4/Kaolin: Synthesis, Performance and Optimization with Response Surface Methodology. Nanomaterials 2020, 10, 1370. [Google Scholar] [CrossRef]
- Munyeza, C.F.; Osano, A.; Maghanga, J.K.; Forbes, P.B. Polycyclic Aromatic Hydrocarbon Gaseous Emissions from Household Cooking Devices: A Kenyan Case Study. Environ. Toxicol. Chem. 2019, 39, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Hardonnière, K.; Saunier, E.; Lemarié, A.; Fernier, M.; Gallais, I.; Héliès-Toussaint, C.; Mograbi, B.; Antonio, S.; Bénit, P.; Rustin, P.; et al. The environmental car-cinogen benzo[a]pyrene induces a Warburg-like metabolic reprogramming dependent on NHE1 and associated with cell survival. Sci. Rep. 2016, 6, 30776. [Google Scholar] [CrossRef] [PubMed]
- Arkas, M.; Eleades, L.; Paleos, C.M.; Tsiourvas, D. Alkylated hyperbranched polymers as molecular nanosponges for the purification of water from polycyclic aromatic hydrocarbons. J. Appl. Polym. Sci. 2005, 97, 2299–2305. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Onukwuli, O.D.; Ighalo, J.O.; Okoye, P.U. Adsorption of Cationic Dyes on Dacryodes edulis Seeds Activated Carbon Modified Using Phosphoric Acid and Sodium Chloride. Environ. Process. 2020, 7, 1151–1171. [Google Scholar] [CrossRef]
- Mudhoo, A.; Gautam, R.K.; Ncibi, M.C.; Zhao, F.; Garg, V.K.; Sillanpää, A. Green synthesis, activation and functionalization of adsorbents for dye sequestration. Environ. Chem. Lett. 2019, 17, 157–193. [Google Scholar] [CrossRef]
- Wazir, M.B.; Daud, M.; Ali, F.; Al-Harthi, M.A. Dendrimer assisted dye-removal: A critical review of adsorption and catalytic degradation for wastewater treatment. J. Mol. Liq. 2020, 315, 113775. [Google Scholar] [CrossRef]
- Hayati, B.; Mahmoodi, N.M.; Arami, M.; Mazaheri, F. Dye Removal from Colored Textile Wastewater by Poly(propylene imine) Dendrimer: Operational Parameters and Isotherm Studies. CLEAN—Soil Air Water 2011, 39, 673–679. [Google Scholar] [CrossRef]
- Kutz, A.; Mariani, G.; Schweins, R.; Streb, C.; Gröhn, F. Self-assembled polyoxometalate–dendrimer structures for selective photo-catalysis. Nanoscale 2018, 10, 914–920. [Google Scholar] [CrossRef]
- Cui, C.; Xie, Y.-D.; Niu, J.-J.; Hu, H.-L.; Lin, S. Poly(Amidoamine) Dendrimer Modified Superparamagnetic Nanoparticles as an Efficient Adsorbent for Cr(VI) Removal: Effect of High-Generation Dendrimer on Adsorption Performance. J. Inorg. Organomet. Polym. Mater. 2022, 32, 840–853. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, B.; He, S.; Man, R. Preparation of Graphene-Oxide/Polyamidoamine Dendrimers and Their Adsorption Properties toward Some Heavy Metal Ions. J. Chem. Eng. Data 2014, 59, 1719–1726. [Google Scholar] [CrossRef]
- Lotfi, Z.; Mousavi, H.Z.; Sajjadi, S.M. Covalently bonded dithiocarbamate-terminated hyperbranched polyamidoamine polymer on magnetic graphene oxide nanosheets as an efficient sorbent for preconcentration and separation of trace levels of some heavy metal ions in food samples. J. Food Meas. Charact. 2019, 14, 293–302. [Google Scholar] [CrossRef]
- Beraa, A.; Hajjaji, M.; Laurent, R.; Delavaux-Nicot, B.; Caminade, A.-M. Removal of chromate from aqueous solutions by dendrimers-clay nanocomposites. Desalin. Water Treat. 2016, 57, 14290–14303. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Li, X.-N.; Wang, C.-Z.; Kong, X.; Zhoug, L.-Z. Poly(styrene-co-divinylbenzene)-PAMAM-IDA chelating resin: Synthesis, characterization and application for Ni(II) removal in aqueous. J. Cent. South Univ. 2014, 21, 3479–3484. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; He, X.; Xiao, M.; Zhang, W.; Lu, C. A super biosorbent from dendrimer poly(amidoamine)-grafted cellulose nanofibril aerogels for effective removal of Cr(vi). J. Mater. Chem. A 2015, 3, 14703–14711. [Google Scholar] [CrossRef]
- Han, K.N.; Yu, B.Y.; Kwak, S.-Y. Hyperbranched poly(amidoamine)/polysulfone composite membranes for Cd(II) removal from water. J. Membr. Sci. 2012, 396, 83–91. [Google Scholar] [CrossRef]
- Barakat, M.; Ramadan, M.; Kuhn, J.; Woodcock, H. Equilibrium and kinetics of Pb2+ adsorption from aqueous solution by dendrimer/titania composites. Desalin. Water Treat. 2013, 52, 5869–5875. [Google Scholar] [CrossRef]
- Liang, X.; Ge, Y.; Wu, Z.; Qin, W. DNA fragments assembled on polyamidoamine-grafted core-shell magnetic silica nanoparticles for removal of mercury(II) and methylmercury(I). J. Chem. Technol. Biotechnol. 2016, 92, 819–826. [Google Scholar] [CrossRef]
- Diallo, M.S.; Christie, S.; Swaminathan, P.; Johnson, J.H.; Goddard, W.A. Dendrimer Enhanced Ultrafiltration. 1. Recovery of Cu(II) from Aqueous Solutions Using PAMAM Dendrimers with Ethylene Diamine Core and Terminal NH2 Groups. Environ. Sci. Technol. 2005, 39, 1366–1377. [Google Scholar] [CrossRef] [Green Version]
- Hayati, B.; Arami, M.; Maleki, A.; Pajootan, E. Application of dendrimer/titania nanohybrid for the removal of phenol from contaminated wastewater. Desalin.Water Treat. 2016, 57, 6809–6819. [Google Scholar] [CrossRef]
- Hayati, B.; Maleki, A.; Najafi, F.; Daraei, H.; Gharibi, F.; McKay, G. Super high removal capacities of heavy metals (Pb2+ and Cu2+) using CNT dendrimer. J. Hazard. Mater. 2017, 336, 146–157. [Google Scholar] [CrossRef]
- Almasian, A.; Olya, M.E.; Mahmoodi, N.M. Synthesis of polyacrylonitrile/polyamidoamine composite nanofibers using electrospinning technique and their dye removal capacity. J. Taiwan Inst. Chem. Eng. 2015, 49, 119–128. [Google Scholar] [CrossRef]
- Salimpour Abkenar, S.; Malek, R.M.A.; Mazaheri, F. Dye adsorption of cotton fabric grafted with PPI dendrimers: Isotherm and kinetic studies. J. Environ. Manag. 2015, 163, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ghasempour, A.; Pajootan, E.; Bahrami, H.; Arami, M. Introduction of amine terminated dendritic structure to graphene oxide using Poly(propylene Imine) dendrimer to evaluate its organic contaminant removal. J. Taiwan Inst. Chem. Eng. 2017, 71, 285–297. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M. Functional Dendrimeric “Nanosponges” for the Removal of Polycyclic Aromatic Hydrocarbons from Water. Chem. Mater. 2003, 15, 2844–2847. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Khan, A.; Khan, A.A.P.; Asiri, A.M. Chapter 19—Aerogel applications and future aspects. In Advances in Aerogel Composites for Environmental Remediation; Khan, A.A.P., Ansari, M.O., Khan, A., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 357–367. [Google Scholar]
- Paraskevopoulou, P.; Chriti, D.; Raptopoulos, G.; Anyfantis, G.C. Synthetic Polymer Aerogels in Particulate Form. Materials 2019, 12, 1543. [Google Scholar] [CrossRef] [Green Version]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Polymeric Hydrogels: Characterization and Biomedical Applications. Des. Monomers Polym. 2009, 12, 197–220. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Su, Y.; Wang, W.; Fang, Y.; Riffat, S.B.; Jiang, F. The advances of polysaccharide-based aerogels: Preparation and potential application. Carbohydr. Polym. 2019, 226, 115242. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- Mekonnen, B.T.; Ding, W.; Liu, H.; Guo, S.; Pang, X.; Ding, Z.; Seid, M.H. Preparation of aerogel and its application progress in coatings: A mini overview. J. Leather Sci. Eng. 2021, 3, 25. [Google Scholar] [CrossRef]
- García-González, C.; Camino-Rey, M.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- Guastaferro, M.; Reverchon, E.; Baldino, L. Polysaccharide-Based Aerogel Production for Biomedical Applications: A Comparative Review. Materials 2021, 14, 1631. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Li, L.; Zhang, P. Macroporous MnO2-based aerogel crosslinked with cellulose nanofibers for efficient ozone removal under humid condition. J. Hazard. Mater. 2020, 407, 124793. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.; Li, Y.; Ma, J. Environmental application and design of alginate/graphene double-network nanocomposite beads. New Polym. Nanocompos. Environ. Remediat. 2018, 2018, 47–76. [Google Scholar] [CrossRef]
- Maleki, H.; Hüsing, N. 16—Aerogels as promising materials for environmental remediation—A broad insight into the envi-ronmental pollutants removal through adsorption and (photo)catalytic processes. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 389–436. [Google Scholar]
- Chhetri, K.; Subedi, S.; Muthurasu, A.; Ko, T.H.; Dahal, B.; Kim, H.Y. A review on nanofiber reinforced aerogels for energy storage and conversion applications. J. Energy Storage 2022, 46, 103927. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Ramin, S. Fabrication of starch-graft-poly(acrylamide)/graphene oxide/hydroxyapatite nanocomposite hydrogel adsorbent for removal of malachite green dye from aqueous solution. Int. J. Biol. Macromol. 2018, 106, 101–115. [Google Scholar] [CrossRef]
- Saraf, P.; Abdollahi Movaghar, M.; Montazer, M.; Mahmoudi Rad, M. Bio and photoactive starch/MnO2 and starch/MnO2/cotton hydrogel nanocomposite. Int. J. Biol. Macromol. 2021, 193, 681–692. [Google Scholar] [CrossRef]
- Herman, P.; Kiss, A.; Fábián, I.; Kalmár, J.; Nagy, G. In situ remediation efficacy of hybrid aerogel adsorbent in model aquatic culture of Paramecium caudatum exposed to Hg(II). Chemosphere 2021, 275, 130019. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; He, X.; Zhang, W.; Zhang, X.; Lu, C. In situ synthesis of MnO2 coated cellulose nanofibers hybrid for effective removal of methylene blue. Carbohydr. Polym. 2014, 110, 302–308. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Al-Deyab, S.S. Preparation of poly(acrylic acid)/starch hydrogel and its application for cadmium ion removal from aqueous solutions. React. Funct. Polym. 2014, 75, 1–8. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Yuan, B.; Fu, M.-L. A facile foaming-polymerization strategy to prepare 3D MnO2 modified biochar-based porous hydrogels for efficient removal of Cd(II) and Pb(II). Chemosphere 2019, 239, 124745. [Google Scholar] [CrossRef] [PubMed]
- Arayaphan, J.; Maijan, P.; Boonsuk, P.; Chantarak, S. Synthesis of photodegradable cassava starch-based double network hydrogel with high mechanical stability for effective removal of methylene blue. Int. J. Biol. Macromol. 2020, 168, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Jindal, R. Comparative study on the behaviour of Chitosan-Gelatin based Hydrogel and nanocomposite ion exchanger synthesized under microwave conditions towards photocatalytic removal of cationic dyes. Carbohydr. Polym. 2018, 207, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Zhu, H.-Y.; Fu, Y.-Q.; Jiang, S.-T.; Zong, E.-M.; Zhu, J.-Q. Colloidal CdS sensitized nano-ZnO/chitosan hydrogel with fast and efficient photocatalytic removal of congo red under solar light irradiation. Int. J. Biol. Macromol. 2021, 174, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.S.; Wang, X.; Abbasi, M.S.; Arshad, N.; Chen, Z.; Guo, Z. Semiconductive, Flexible MnO2 NWs/Chitosan Hydrogels for Efficient Solar Steam Generation. ACS Sustain. Chem. Eng. 2021, 9, 3887–3900. [Google Scholar] [CrossRef]
- Adeola, A.O.; Abiodun, B.A.; Adenuga, D.O.; Nomngongo, P.N. Adsorptive and photocatalytic remediation of hazardous organic chemical pollutants in aqueous medium: A review. J. Contam. Hydrol. 2022, 248, 104019. [Google Scholar] [CrossRef]
- Ray, P.; Singh, P.S.; Polisetti, V. 2—Synthetic polymeric membranes for the removal of toxic pollutants and other harmful contaminants from water. In Removal of Toxic Pollutants through Microbiological and Tertiary Treatment; Shah, M.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 43–99. [Google Scholar]
- Mukherjee, M.; Roy, S.; Bhowmick, K.; Majumdar, S.; Prihatiningtyas, I.; Van der Bruggen, B.; Mondal, P. Development of high performance pervaporation desalination membranes: A brief review. Process Saf. Environ. Prot. 2022, 159, 1092–1104. [Google Scholar] [CrossRef]
- Wilson, R.; George, G.; Jose, A.J. Polymer membranes reinforced with carbon-based nanomaterials for water purification. New Polym. Nanocompos. Environ. Remediat. 2018, 2018, 457–468. [Google Scholar] [CrossRef]
- Potara, M.; Focsan, M.; Craciun, A.-M.; Botiz, I.; Astilean, S. 15—Polymer-coated plasmonic nanoparticles for environmental reme-diation: Synthesis, functionalization, and properties. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 361–387. [Google Scholar]
- Leudjo Taka, A.; Klink, M.J.; Yangkou Mbianda, X.; Naidoo, E.B. Chitosan nanocomposites for water treatment by fixed-bed con-tinuous flow column adsorption: A review. Carbohydr. Polym. 2021, 255, 117398. [Google Scholar] [CrossRef]
- Sapna, K.D. 12—Biodegradable polymer-based nanoadsorbents for environmental remediation. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 261–278. [Google Scholar]
- Semyonov, O.; Kogolev, D.; Mamontov, G.; Kolobova, E.; Trelin, A.; Yusubov, M.S.; Guselnikova, O.; Postnikov, P.S. Synergetic effect of UiO-66 and plasmonic AgNPs on PET waste support towards degradation of nerve agent simulant. Chem. Eng. J. 2021, 431, 133450. [Google Scholar] [CrossRef]
- Fahma, F.; Febiyanti, I.; Lisdayana, N.; Arnata, I.; Sartika, D. Nanocellulose as a new sustainable material for various applications: A review. Arch. Mater. Sci. Eng. 2021, 2, 49–64. [Google Scholar] [CrossRef]
- Iba, H.; Chang, T.; Kagawa, Y. Optically transparent continuous glass fibre-reinforced epoxy matrix composite: Fabrication, optical and mechanical properties. Compos. Sci. Technol. 2002, 62, 2043–2052. [Google Scholar] [CrossRef]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2018, 88, 241–264. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Etale, A.; Wang, J.; Berglund, L.A.; Li, Y.; Cai, Y.; Chen, C.; Cranston, E.D.; Johns, M.A.; Fang, Z.; et al. Current international research into cellulose as a functional nanomaterial for advanced applications. J. Mater. Sci. 2022, 57, 5697–5767. [Google Scholar] [CrossRef]
- Adam, M.R.; Hubadillah, S.K.; Esham, M.I.M.; Othman, M.H.D.; Rahman, M.A.; Ismail, A.F. Chapter 12—Adsorptive Membranes for Heavy Metals Removal From Water. In Membrane Separation Principles and Applications; Ismail, A.F., Rahman, M.A., Othman, M.H.D., Matsuura, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 361–400. [Google Scholar]
- Zhang, X.; Fang, X.; Li, J.; Pan, S.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Zhao, S. Developing new adsorptive membrane by modification of support layer with iron oxide microspheres for arsenic removal. J. Colloid Interface Sci. 2018, 514, 760–768. [Google Scholar] [CrossRef]
- Tabrizi, S.H.; Tanhaei, B.; Ayati, A.; Ranjbari, S. Substantial improvement in the adsorption behavior of montmorillonite toward Tartrazine through hexadecylamine impregnation. Environ. Res. 2021, 204, 111965. [Google Scholar] [CrossRef] [PubMed]
- Qalyoubi, L.; Al-Othman, A.; Al-Asheh, S. Recent progress and challenges on adsorptive membranes for the removal of pollutants from wastewater. Part I: Fundamentals and classification of membranes. Case Stud. Chem. Environ. Eng. 2021, 3, 100086. [Google Scholar] [CrossRef]
- Salehi, E.; Daraei, P.; Shamsabadi, A.A. A review on chitosan-based adsorptive membranes. Carbohydr. Polym. 2016, 152, 419–432. [Google Scholar] [CrossRef]
- Qadir, D.; Nasir, R.; Mukhtar, H.B.; Keong, L.K. Synthesis, characterization, and performance analysis of carbon molecular sieve-embedded polyethersulfone mixed-matrix membranes for the removal of dissolved ions. Water Environ. Res. 2020, 92, 1306–1324. [Google Scholar] [CrossRef]
- Kang, M.; Kawasaki, M.; Tamada, S.; Kamei, T.; Magara, Y. Effect of pH on the removal of arsenic and antimony using reverse osmosis membranes. Desalination 2000, 131, 293–298. [Google Scholar] [CrossRef]
- Oh, J.; Yamamoto, K.; Kitawaki, H.; Nakao, S.; Sugawara, T.; Rahman, M. Application of low-pressure nanofiltration coupled with a bicycle pump for the treatment of arsenic-contaminated groundwater. Desalination 2000, 132, 307–314. [Google Scholar] [CrossRef]
- Mojarrad, M.; Noroozi, A.; Zeinivand, A.; Kazemzadeh, P. Response surface methodology for optimization of simultaneous Cr (VI) and as (V) removal from contaminated water by nanofiltration process. Environ. Prog. Sustain. Energy 2017, 37, 434–443. [Google Scholar] [CrossRef]
- He, Y.; Tang, Y.P.; Ma, D.; Chung, T.-S. UiO-66 incorporated thin-film nanocomposite membranes for efficient selenium and arsenic removal. J. Membr. Sci. 2017, 541, 262–270. [Google Scholar] [CrossRef]
- He, Y.; Liu, J.; Han, G.; Chung, N.T.-S. Novel thin-film composite nanofiltration membranes consisting of a zwitterionic co-polymer for selenium and arsenic removal. J. Membr. Sci. 2018, 555, 299–306. [Google Scholar] [CrossRef]
- Manirethan, V.; Gupta, N.; Balakrishnan, R.M.; Raval, K. Batch and continuous studies on the removal of heavy metals from aqueous solution using biosynthesised melanin-coated PVDF membranes. Environ. Sci. Pollut. Res. 2019, 27, 24723–24737. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Kwak, S.-Y. Surface functionalization of PTFE membranes with hyperbranched poly(amidoamine) for the removal of Cu2+ ions from aqueous solution. J. Membr. Sci. 2013, 448, 125–134. [Google Scholar] [CrossRef]
- Weng, Y.-H.; Chaung-Hsieh, L.H.; Lee, H.-H.; Li, K.-C.; Huang, C.P. Removal of arsenic and humic substances (HSs) by elec-tro-ultrafiltration (EUF). J. Hazard. Mater. 2005, 122, 171–176. [Google Scholar] [CrossRef]
- Cai, Y.-H.; Yang, X.J.; Schäfer, A.I. Removal of Naturally Occurring Strontium by Nanofiltration/Reverse Osmosis from Ground-water. Membranes 2020, 10, 321. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, C.; Tong, T.; Epsztein, R.; Sun, M.; Verduzco, R. Selective removal of divalent cations by polyelectrolyte mul-tilayer nanofiltration membrane: Role of polyelectrolyte charge, ion size, and ionic strength. J. Membr. Sci. 2018, 559, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Fakhfekh, R.; Chabanon, E.; Mangin, D.; Ben Amar, R.; Charcosset, C. Removal of iron using an oxidation and ceramic microfil-tration hybrid process for drinking water treatment. Desalin. Water Treat. 2017, 66, 210–220. [Google Scholar] [CrossRef]
- Luo, L.; Han, G.; Chung, T.-S.; Weber, M.; Staudt, C.; Maletzko, C. Oil/water separation via ultrafiltration by novel triangle-shape tri-bore hollow fiber membranes from sulfonated polyphenylenesulfone. J. Membr. Sci. 2014, 476, 162–170. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, Z.; Xie, A.; Wang, Q.; Liu, S.; Lang, J. Facile preparation of grass-like structured NiCo-LDH/PVDF composite membrane for efficient oil–water emulsion separation. J. Membr. Sci. 2019, 573, 226–233. [Google Scholar] [CrossRef]
- Cui, J.; Xie, A.; Zhou, S.; Liu, S.; Wang, Q.; Wu, Y.; Meng, M.; Lang, J.; Zhou, Z.; Yan, Y. Development of composite membranes with irregular rod-like structure via atom transfer radical polymerization for efficient oil-water emulsion separation. J. Colloid Interface Sci. 2018, 533, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ding, Y.; Wang, J.; Li, T.; Lin, H.; Wang, J.; Liu, F. Facile fabrication of nanofiber- and micro/nanosphere-coordinated PVDF membrane with ultrahigh permeability of viscous water-in-oil emulsions. J. Mater. Chem. A 2018, 6, 7014–7020. [Google Scholar] [CrossRef]
- Zioui, D.; Salazar, H.; Aoudjit, L.; Martins, P.M.; Lanceros-Méndez, S. Polymer-Based Membranes for Oily Wastewater Remediation. Polymers 2020, 12, 42. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.; Dai, F.; Lin, L.; An, Z.; He, Y.; Chen, X.; Chen, L.; Zhao, Y. Simplified and robust adhesive-free superhydrophobic SiO2-decorated PVDF membranes for efficient oil/water separation. J. Membr. Sci. 2018, 555, 220–228. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abidin, Z.Z.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S. Application of membrane-coupled se-quencing batch reactor for oilfield produced water recycle and beneficial re-use. Bioresour. Technol. 2010, 101, 6942–6949. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Chen, L.; Feng, Y.; He, W.; Lv, X. Modified superhydrophilic and underwater superoleophobic PVDF membrane with ultralow oil-adhesion for highly efficient oil/water emulsion separation. Mater. Lett. 2016, 185, 169–172. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Pan, Y.; Di, H.; Zeng, G.; Zhang, L. A modified mussel-inspired method to fabricate TiO2 decorated superhy-drophilic PVDF membrane for oil/water separation. J. Membr. Sci. 2016, 506, 60–70. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Yap, P.-S.; Iwuozor, K.O.; Aniagor, C.O.; Liu, T.; Dulta, K.; Iwuchukwu, F.U.; Rangabhashiyam, S. Adsorption of persistent organic pollutants (POPs) from the aqueous environment by nano-adsorbents: A review. Environ. Res. 2022, 212, 113123. [Google Scholar] [CrossRef]
- Peng, B.L.; Yao, Z.L.; Wang, X.C.; Crombeen, M.; Sweeney, D.G.; Tam, K.C. Cellulose-based materials in wastewater treatment of petroleum industry. Green Energy Environ. 2020, 5, 37–49. [Google Scholar] [CrossRef]
- Adeola, A.O.; Kubheka, G.; Chirwa, E.M.N.; Forbes, P.B.C. Facile synthesis of graphene wool doped with oleylamine-capped silver nanoparticles (GW-αAgNPs) for water treatment applications. Appl. Water Sci. 2021, 11, 172. [Google Scholar] [CrossRef]
- Sajid, M.; Ilyas, M.; Basheer, C.; Tariq, M.; Daud, M.; Baig, N.; Shehzad, F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut. Res. 2014, 22, 4122–4143. [Google Scholar] [CrossRef] [PubMed]
- Bhoj, Y.; Tharmavaram, M.; Rawtani, D. A comprehensive approach to antifouling strategies in desalination, marine environment, and wastewater treatment. Chem. Phys. Impact 2020, 2, 100008. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeola, A.O.; Nomngongo, P.N. Advanced Polymeric Nanocomposites for Water Treatment Applications: A Holistic Perspective. Polymers 2022, 14, 2462. https://doi.org/10.3390/polym14122462
Adeola AO, Nomngongo PN. Advanced Polymeric Nanocomposites for Water Treatment Applications: A Holistic Perspective. Polymers. 2022; 14(12):2462. https://doi.org/10.3390/polym14122462
Chicago/Turabian StyleAdeola, Adedapo Oluwasanu, and Philiswa Nosizo Nomngongo. 2022. "Advanced Polymeric Nanocomposites for Water Treatment Applications: A Holistic Perspective" Polymers 14, no. 12: 2462. https://doi.org/10.3390/polym14122462
APA StyleAdeola, A. O., & Nomngongo, P. N. (2022). Advanced Polymeric Nanocomposites for Water Treatment Applications: A Holistic Perspective. Polymers, 14(12), 2462. https://doi.org/10.3390/polym14122462