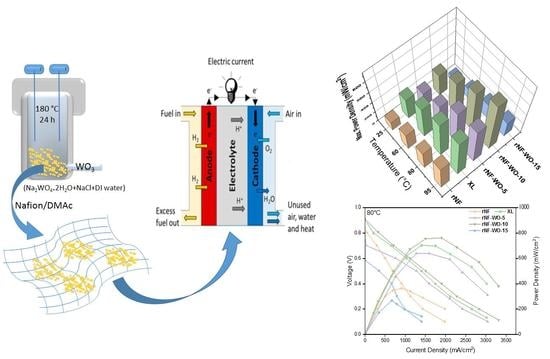
Development of WO3–Nafion Based Membranes for Enabling Higher Water Retention at Low Humidity and Enhancing PEMFC Performance at Intermediate Temperature Operation
Abstract
:1. Introduction
2. Experimental Methodology
2.1. Materials
2.2. Synthesis of WO3 Nanoparticles
2.3. Preparation of Nafion Nanocomposite Membranes
2.4. Membrane Characterisation
2.5. MEA Fabrication and Fuel Cell Tests
2.5.1. Catalyst Preparation
2.5.2. Fuel Cell Tests
3. Results and Discussion
3.1. Morphology and Properties
3.2. Contact Angle, Water Uptake, Swelling Ratio, Ion Exchange Capacity, and Hydration Degree
3.3. Membrane Conductivity and In Situ Single Cell Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teixeira, F.C.; de Sá, A.I.; Teixeira, A.P.S.; Ortiz-Martínez, V.M.; Ortiz, A.; Ortiz, I.; Rangel, C.M. New Modified Nafion-Bisphosphonic Acid Composite Membranes for Enhanced Proton Conductivity and PEMFC Performance. Int. J. Hydrogen Energy 2021, 46, 17562–17571. [Google Scholar] [CrossRef]
- Teixeira, F.C.; de Sá, A.I.; Teixeira, A.P.S.; Rangel, C.M. Nafion Phosphonic Acid Composite Membranes for Proton Exchange Membranes Fuel Cells. Appl. Surf. Sci. 2019, 487, 889–897. [Google Scholar] [CrossRef] [Green Version]
- Amjadi, M.; Rowshanzamir, S.; Peighambardoust, S.J.; Sedghi, S. Preparation, Characterization and Cell Performance of Durable Nafion/SiO2 Hybrid Membrane for High-Temperature Polymeric Fuel Cells. J. Power Sources 2012, 210, 350–357. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Herve Bang, Y.; Di Noto, V. Hybrid Inorganic-Organic Proton-Conducting Membranes Based on SPEEK Doped with WO3 Nanoparticles for Application in Vanadium Redox Flow Batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Ramya, K.; Khalid, M.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Walvekar, R.; Kadhum, A.A.H. Additives in Proton Exchange Membranes for Low- and High-Temperature Fuel Cell Applications: A Review. Int. J. Hydrogen Energy 2019, 44, 6116–6135. [Google Scholar] [CrossRef]
- Prykhodko, Y.; Fatyeyeva, K.; Hespel, L.; Marais, S. Progress in Hybrid Composite Nafion®-Based Membranes for Proton Exchange Fuel Cell Application. Chem. Eng. J. 2021, 409, 127329. [Google Scholar] [CrossRef]
- Mahato, N.; Jang, H.; Dhyani, A.; Cho, S. Recent Progress in Conducting Polymers for Hydrogen Storage and Fuel Cell Applications. Polymers 2020, 12, 2480. [Google Scholar] [CrossRef]
- Alanazi, A.; Ogungbemi, E.; Wilberforce, A.; Awotwe, T.W.; Ijaodola, O.; Vichare, P.; Olabi, A.G. State-of-the-Art Manufacturing Technologies of PEMFC Components; University of the West of Scotland: London, UK, 2017; pp. 189–198. [Google Scholar] [CrossRef] [Green Version]
- Goh, J.T.E.; Rahim, A.R.A.; Masdar, M.S.; Shyuan, L.K. Enhanced Performance of Polymer Electrolyte Membranes via Modification with Ionic Liquids for Fuel Cell Applications. Membranes 2021, 11, 395. [Google Scholar] [CrossRef]
- de Bruijn, F.A.; Makkus, R.C.; Mallant, R.K.A.M.; Janssen, G.J.M. Chapter Five Materials for State-of-the-Art PEM Fuel Cells, and Their Suitability for Operation Above 100°C. Adv. Fuel Cells 2007, 1, 235–336. [Google Scholar]
- Rhee, H.W.; Ghil, L.J. Polymer Nanocomposites in Fuel Cells; Woodhead Publishing Limited: Sawston, UK, 2012; ISBN 9781845699406. [Google Scholar]
- Prasad, M.; Mohanty, S.; Nayak, S.K. Polymer Electrolyte Membranes Based on Sulfonated Polysulfone and Functionalized Layered Silicate for Direct Methanol Fuel Cell Applications. High Perform. Polym. 2015, 27, 714–723. [Google Scholar] [CrossRef]
- Asgari, M.S.; Nikazar, M.; Molla-Abbasi, P.; Hasani-Sadrabadi, M.M. Nafion®/Histidine Functionalized Carbon Nanotube: High-Performance Fuel Cell Membranes. Int. J. Hydrogen Energy 2013, 38, 5894–5902. [Google Scholar] [CrossRef]
- Sasikala, S.; Meenakshi, S.; Bhat, S.D.; Sahu, A.K. Functionalized Bentonite Clay-SPEEK Based Composite Membranes for Direct Methanol Fuel Cells. Electrochim. Acta 2014, 135, 232–241. [Google Scholar] [CrossRef]
- Mahmoud, A.M.A.; Yoshimura, K.; Maekawa, Y. Alkaline Fuel Cells Consisting of Imidazolium-Based Graft-Type Anion Exchange Membranes: Optimization of Fuel Cell Conditions to Achieve High Performance and Durability. J. Memb. Sci. 2021, 620, 118844. [Google Scholar] [CrossRef]
- Chu, X.; Liu, J.; Miao, S.; Liu, L.; Huang, Y.; Tang, E.; Liu, S.; Xing, X.; Li, N. Crucial Role of Side-Chain Functionality in Anion Exchange Membranes: Properties and Alkaline Fuel Cell Performance. J. Memb. Sci. 2021, 625, 119172. [Google Scholar] [CrossRef]
- Ho, J.H.; Li, Y.; Dai, Y.; Kim, T.I.; Wang, J.; Ren, J.; Yun, H.S.; Liu, X. Ionothermal Synthesis of N-Doped Carbon Supported CoMn2O4 Nanoparticles as ORR Catalyst in Direct Glucose Alkaline Fuel Cell. Int. J. Hydrogen Energy 2021, 46, 20503–20515. [Google Scholar] [CrossRef]
- Peng, J.; Huang, J.; Wu, X.L.; Xu, Y.W.; Chen, H.; Li, X. Solid Oxide Fuel Cell (SOFC) Performance Evaluation, Fault Diagnosis and Health Control: A Review. J. Power Sources 2021, 505, 230058. [Google Scholar] [CrossRef]
- Singh, M.; Zappa, D.; Comini, E. Solid Oxide Fuel Cell: Decade of Progress, Future Perspectives and Challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Effect of Deep Eutectic Solvents Hydrogen Bond Acceptor on the Anhydrous Proton Conductivity of Nafion Membrane for Fuel Cell Applications. J. Memb. Sci. 2020, 605, 118116. [Google Scholar] [CrossRef]
- Amjadi, M.; Rowshanzamir, S.; Peighambardoust, S.J.; Hosseini, M.G.; Eikani, M.H. Investigation of Physical Properties and Cell Performance of Nafion/TiO2 Nanocomposite Membranes for High Temperature PEM Fuel Cells. Int. J. Hydrogen Energy 2010, 35, 9252–9260. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Tu, Z.; Yu, J.; Xiong, C.; Pan, M. Impregnation of Amine-Tailored Titanate Nanotubes in Polymer Electrolyte Membranes. J. Memb. Sci. 2012, 423–424, 284–292. [Google Scholar] [CrossRef]
- Chia, M.Y.; Thiam, H.S.; Leong, L.K.; Koo, C.H.; Saw, L.H. Study on Improvement of the Selectivity of Proton Exchange Membrane via Incorporation of Silicotungstic Acid-Doped Silica into SPEEK. Int. J. Hydrogen Energy 2020, 45, 22315–22323. [Google Scholar] [CrossRef]
- Sahin, A. The Development of Speek/Pva/Teos Blend Membrane for Proton Exchange Membrane Fuel Cells. Electrochim. Acta 2018, 271, 127–136. [Google Scholar] [CrossRef]
- Hooshyari, K.; Rezania, H.; Vatanpour, V.; Salarizadeh, P.; Askari, M.B.; Beydaghi, H.; Enhessari, M. High Temperature Membranes Based on PBI/Sulfonated Polyimide and Doped-Perovskite Nanoparticles for PEM Fuel Cells. J. Memb. Sci. 2020, 612, 118436. [Google Scholar] [CrossRef]
- Moradi, M.; Moheb, A.; Javanbakht, M.; Hooshyari, K. Experimental Study and Modeling of Proton Conductivity of Phosphoric Acid Doped PBI-Fe2TiO5 Nanocomposite Membranes for Using in High Temperature Proton Exchange Membrane Fuel Cell (HT-PEMFC). Int. J. Hydrogen Energy 2016, 41, 2896–2910. [Google Scholar] [CrossRef]
- Gouda, M.H.; Elessawy, N.A.; Toghan, A. Development of Effectively Costed and Performant Novel Cation Exchange Ceramic Nanocomposite Membrane Based Sulfonated PVA for Direct Borohydride Fuel Cells. J. Ind. Eng. Chem. 2021, 100, 212–219. [Google Scholar] [CrossRef]
- Pagidi, A.; Arthanareeswaran, G.; Seepana, M.M. Synthesis of Highly Stable PTFE-ZrP-PVA Composite Membrane for High-Temperature Direct Methanol Fuel Cell. Int. J. Hydrogen Energy 2020, 45, 7829–7837. [Google Scholar] [CrossRef]
- Malik, R.S.; Soni, U.; Chauhan, S.S.; Kumar, D.; Choudhary, V. Semi-Interpenetrating Polymer Networks of Poly (Vinyl Alcohol)-Functionalized Nanocrystals/Sulfonated Poly (Ether Ether Ketone) (PVA-FNCs/SPEEK) as Fuel Cell Membrane. Mater. Today Commun. 2021, 29, 102897. [Google Scholar] [CrossRef]
- He, Y.; Wang, D.; Li, Q.; Huang, L.; Bao, H. Composite Polymer Electrolyte Membranes Based on Nafion and Modified PVDF Electrospun Nanofiber Mats. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2020, 35, 677–681. [Google Scholar] [CrossRef]
- Yagizatli, Y.; Ulas, B.; Cali, A.; Sahin, A.; Ar, I. Improved Fuel Cell Properties of Nano-TiO2 Doped Poly(Vinylidene Fluoride) and Phosphonated Poly(Vinyl Alcohol) Composite Blend Membranes for PEM Fuel Cells. Int. J. Hydrogen Energy 2020, 45, 35130–35138. [Google Scholar] [CrossRef]
- Bagus Pambudi, A.; Priyangga, A.; Hartanto, D.; Atmaja, L. Fabrication and Characterization of Modified Microcrystalline Cellulose Membrane as Proton Exchange Membrane for Direct Methanol Fuel Cell. Mater. Today Proc. 2020, 46, 1855–1859. [Google Scholar] [CrossRef]
- Sun, C.; Zlotorowicz, A.; Nawn, G.; Negro, E.; Bertasi, F.; Pagot, G.; Vezzù, K.; Pace, G.; Guarnieri, M.; Di Noto, V. [Nafion/(WO3)x] Hybrid Membranes for Vanadium Redox Flow Batteries. Solid State Ionics 2018, 319, 110–116. [Google Scholar] [CrossRef]
- Schalenbach, M.; Hoefner, T.; Paciok, P.; Carmo, M.; Lueke, W.; Stolten, D. Gas Permeation through Nafion. Part 1 Measurements. J. Phys. Chem. C 2015, 119, 25145–25155. [Google Scholar] [CrossRef]
- Schalenbach, M.; Hoeh, M.A.; Gostick, J.T.; Lueke, W.; Stolten, D. Gas Permeation through Nafion. Part 2: Resistor Network Model. J. Phys. Chem. C 2015, 119, 25156–25169. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef]
- Vishnyakov, A.; Neimark, A.V. Self-Assembly in Nafion Membranes upon Hydration: Water Mobility and Adsorption Isotherms. J. Phys. Chem. B 2014, 118, 11353–11364. [Google Scholar] [CrossRef]
- Wang, Y.; Ruiz Diaz, D.F.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, Technological Status, and Fundamentals of PEM Fuel Cells – A Review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Sridhar, P.; Perumal, R.; Rajalakshmi, N.; Raja, M.; Dhathathreyan, K.S. Humidification Studies on Polymer Electrolyte Membrane Fuel Cell. J. Power Sources 2001, 101, 72–78. [Google Scholar] [CrossRef]
- He, X.; He, G.; Zhao, A.; Wang, F.; Mao, X.; Yin, Y.; Cao, L.; Zhang, B.; Wu, H.; Jiang, Z. Facilitating Proton Transport in Nafion-Based Membranes at Low Humidity by Incorporating Multifunctional Graphene Oxide Nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 27676–27687. [Google Scholar] [CrossRef]
- Goo, B.H.; Paek, S.Y.; Al Munsur, A.Z.; Choi, O.; Kim, Y.; Kwon, O.J.; Lee, S.Y.; Kim, H.J.; Kim, T.H. Polyamide-Coated Nafion Composite Membranes with Reduced Hydrogen Crossover Produced via Interfacial Polymerization. Int. J. Hydrogen Energy 2022, 47, 1202–1216. [Google Scholar] [CrossRef]
- Haghighi, A.H.; Tohidian, M.; Ghaderian, A.; Shakeri, S.E. Polyelectrolyte Nanocomposite Membranes Using Surface Modified Nanosilica for Fuel Cell Applications. J. Macromol. Sci. Part B Phys. 2017, 56, 383–394. [Google Scholar] [CrossRef]
- Xu, G.; Zou, J.; Guo, Z.; Li, J.; Ma, L.; Li, Y.; Cai, W. Bi-Functional Composting the Sulfonic Acid Based Proton Exchange Membrane for High Temperature Fuel Cell Application. Polymers 2020, 12, 1000. [Google Scholar] [CrossRef] [PubMed]
- Treekamol, Y.; Schieda, M.; Robitaille, L.; MacKinnon, S.M.; Mokrini, A.; Shi, Z.; Holdcroft, S.; Schulte, K.; Nunes, S.P. Nafion®/ODF-Silica Composite Membranes for Medium Temperature Proton Exchange Membrane Fuel Cells. J. Power Sources 2014, 246, 950–959. [Google Scholar] [CrossRef]
- Hong, L.Y.; Oh, S.Y.; Matsuda, A.; Lee, C.S.; Kim, D.P. Hydrophilic and Mesoporous SiO2-TiO2-SO3H System for Fuel Cell Membrane Applications. Electrochim. Acta 2011, 56, 3108–3114. [Google Scholar] [CrossRef]
- Taghizadeh, M.T.; Vatanparast, M. Ultrasonic-Assisted Synthesis of ZrO2 Nanoparticles and Their Application to Improve the Chemical Stability of Nafion Membrane in Proton Exchange Membrane (PEM) Fuel Cells. J. Colloid Interface Sci. 2016, 483, 1–10. [Google Scholar] [CrossRef]
- Aziz, M.A.; Shanmugam, S. Zirconium Oxide Nanotube–Nafion Composite as High Performance Membrane for All Vanadium Redox Flow Battery. J. Power Sources 2017, 337, 36–44. [Google Scholar] [CrossRef]
- Bébin, P.; Caravanier, M.; Galiano, H. Nafion®/Clay-SO3H Membrane for Proton Exchange Membrane Fuel Cell Application. J. Memb. Sci. 2006, 278, 35–42. [Google Scholar] [CrossRef]
- Kim, T.K.; Kang, M.; Choi, Y.S.; Kim, H.K.; Lee, W.; Chang, H.; Seung, D. Preparation of Nafion-Sulfonated Clay Nanocomposite Membrane for Direct Menthol Fuel Cells via a Film Coating Process. J. Power Sources 2007, 165, 1–8. [Google Scholar] [CrossRef]
- Ibrahim, A.; Hossain, O.; Chaggar, J.; Steinberger-Wilckens, R.; El-Kharouf, A. GO-Nafion Composite Membrane Development for Enabling Intermediate Temperature Operation of Polymer Electrolyte Fuel Cell. Int. J. Hydrogen Energy 2020, 45, 5526–5534. [Google Scholar] [CrossRef]
- Zarrin, H.; Higgins, D.; Jun, Y.; Chen, Z.; Fowler, M. Functionalized Graphene Oxide Nanocomposite Membrane for Low Humidity and High Temperature Proton Exchange Membrane Fuel Cells. J. Phys. Chem. C 2011, 115, 20774–20781. [Google Scholar] [CrossRef]
- Yurova, P.A.; Malakhova, V.R.; Gerasimova, E.V.; Stenina, I.A.; Yaroslavtsev, A.B. Nafion/Surface Modified Ceria Hybrid Membranes for Proton Exchange Fuel Cell Application. Polymers 2021, 13, 2513. [Google Scholar] [CrossRef]
- Shao, Z.G.; Xu, H.; Li, M.; Hsing, I.M. Hybrid Nafion-Inorganic Oxides Membrane Doped with Heteropolyacids for High Temperature Operation of Proton Exchange Membrane Fuel Cell. Solid State Ionics 2006, 177, 779–785. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Huang, Y.; Liang, J.; Shen, P.K. Dynamic Conducting Effect of WO3/PFSA Membranes on the Performance of Proton Exchange Membrane Fuel Cells. J. Power Sources 2008, 177, 56–60. [Google Scholar] [CrossRef]
- Shi, S.; Weber, A.Z.; Kusoglu, A. Structure/Property Relationship of Nafion XL Composite Membranes. J. Memb. Sci. 2016, 516, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Xin, L.; Xia, Y.; Dai, L.; Qu, K.; Huang, K.; Fan, Y.; Xu, Z. Advanced Nafion Hybrid Membranes with Fast Proton Transport Channels toward High-Performance Vanadium Redox Flow Battery. J. Memb. Sci. 2021, 624. [Google Scholar] [CrossRef]
- Románszki, L.; Mohos, M.; Telegdi, J.; Keresztes, Z.; Nyikos, L. A Comparison of Contact Angle Measurement Results Obtained on Bare, Treated, and Coated Alloy Samples by Both Dynamic Sessile Drop and Wilhelmy Method. Period. Polytech. Chem. Eng. 2014, 58, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Cheng, Y.; Sun, L.; Ding, M.; Wu, C.; Yuan, D.; Zhao, X.; Xiang, C.; Jia, C. A Green SPEEK/Lignin Composite Membrane with High Ion Selectivity for Vanadium Redox Flow Battery. J. Memb. Sci. 2019, 572, 110–118. [Google Scholar] [CrossRef]
- Yazici, M.S.; Dursun, S.; Borbáth, I.; Tompos, A. Reformate Gas Composition and Pressure Effect on CO Tolerant Pt/Ti0.8Mo0.2O2–C Electrocatalyst for PEM Fuel Cells. Int. J. Hydrogen Energy 2021, 46, 13524–13533. [Google Scholar] [CrossRef]
- Devrim, Y.; Albostan, A. Enhancement of PEM Fuel Cell Performance at Higher Temperatures and Lower Humidities by High Performance Membrane Electrode Assembly Based on Nafion/Zeolite Membrane. Int. J. Hydrogen Energy 2015, 40, 15328–15335. [Google Scholar] [CrossRef]
- Sizov, V.E.; Zefirov, V.V.; Abramchuk, S.S.; Korlyukov, A.A.; Kondratenko, M.S.; Vasil’ev, V.G.; Gallyamov, M.O. Composite Nafion-Based Membranes with Nanosized Tungsten Oxides Prepared in Supercritical Carbon Dioxide. J. Memb. Sci. 2020, 609, 118244. [Google Scholar] [CrossRef]
- Devrim, Y.; Erkan, S.; Baç, N.; Eroglu, I. Nafion/Titanium Silicon Oxide Nanocomposite Membranes for PEM Fuel Cells. Int. J. Energy Res. 2013, 37, 435–442. [Google Scholar] [CrossRef]
- Di Noto, V.; Gliubizzi, R.; Negro, E.; Vittadello, M.; Pace, G.; Lavina, S.; Negro, E.; Vittadello, M.; Conti, F.; Piga, M.; et al. Hybrid Inorganic-Organic Proton Conducting Membranes Based on Nafion and 5 Wt% of MxOy (M = Ti, Zr, Hf, Ta and W). Part I. Synthesis, Properties and Vibrational Studies. J. Power Sources 2008, 53, 56–60. [Google Scholar] [CrossRef]
- Selim, A.; Toth, A.; Fozer, D.; Süvegh, K.; Mizsey, P. Facile Preparation of a Laponite/PVA Mixed Matrix Membrane for Efficient and Sustainable Pervaporative Dehydration of C1–C3 Alcohols. ACS Omega 2020, 5, 32373–32385. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An Efficient Barrier toward Vanadium Crossover in Redox Flow Batteries: The Bilayer [Nafion/(WO3)x] Hybrid Inorganic-Organic Membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Laporta, M.; Pegoraro, M.; Zanderighi, L. Perfluorosulfonated Membrane (Nafion): FT-IR Study of the State of Water with Increasing Humidity. Phys. Chem. Chem. Phys. 1999, 1, 4619–4628. [Google Scholar] [CrossRef]
- Hatel, R.; Baitoul, M. Nanostructured Tungsten Trioxide (Wo3): Synthesis, Structural and Morphological Investigations. J. Phys. Conf. Ser. 2019, 1292. [Google Scholar] [CrossRef]
- Ahmadian, H.; Tehrani, F.S.; Aliannezhadi, M. Hydrothermal Synthesis and Characterization of WO3 Nanostructures: Effects of Capping Agent and PH. Mater. Res. Express 2019, 6, 105024. [Google Scholar] [CrossRef]
- Tehrani, F.S.; Ahmadian, H.; Aliannezhadi, M. Hydrothermal Synthesis and Characterization of WO3 Nanostructures: Effect of Reaction Time. Mater. Res. Express 2020, 7, 015911. [Google Scholar] [CrossRef]
- Románszki, L.; Datsenko, I.; May, Z.; Telegdi, J.; Nyikos, L.; Sand, W. Polystyrene Films as Barrier Layers for Corrosion Protection of Copper and Copper Alloys. Bioelectrochemistry 2014, 97, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Románszki, L.; Mohos, M.; Telegdi, J.; Nyikos, L. Contact Angle Measurement Is an Efficient Tool for the Characterization of Corrosion Pro- Tection Nanolayers on Copper Alloys and Stainless Steel. Proc. Int. Conf. Nanomater. Appl. Prop. 2013, 2, 9–11. [Google Scholar]
- Hydrogen Europe. Hydrogen Europe Strategic Research and Innovation Agenda, Final Draft; IGEM: Boston, MA, USA, 2020. [Google Scholar]
- KIM, J.; Yamasaki, K.; Ishimoto, H.; Takata, Y. Ultrathin Electrolyte Membranes with PFSA-Vinylon Intermediate Layers for PEM Fuel Cells. Polymers 2020, 12, 1730. [Google Scholar] [CrossRef]
- Letsau, T.T.; Govender, P.P.; Msomi, P.F. Imidazolium-Quaternized Poly(2,6-Dimethyl-1,4-Phenylene Oxide)/Zeolitic Imidazole Framework-8 Composite Membrane as Polymer Electrolyte for Fuel-Cell Application. Polymers 2022, 14, 595. [Google Scholar] [CrossRef]
- Azimirad, R.; Naseri, N.; Akhavan, O.; Moshfegh, A.Z. Hydrophilicity variation of WO3 thin films with annealing temperature. J. Phys. D Appl. Phys. 2007, 40, 1134–1137. [Google Scholar] [CrossRef]
- Behbahani, M.A.; Ranjbar, M.; Kameli, P.; Salamati, H. Hydrogen sensing by wet-gasochromic coloring of PdCl2(aq)/WO3 andthe role of hydrophilicity of tungsten oxide films. Sens. Actuators B 2013, 188, 127–136. [Google Scholar]
- Enesca, A.; Duta, A. Tailoring WO3 thin layers using spray pyrolysis technique. Phys. Stat. Sol. 2008, 5, 3499–3502. [Google Scholar]
- Hemati, A.; Allaf, B.M.; Ranjbar, M.; Kameli, P.; Salamati, H. Gasochromic tungsten oxide films with PdCl2 solution as an aqueous Hydrogen catalyst. Sol. Energy Mater. Sol. Cells 2013, 108, 105–112. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Liu, Q.; Li, Y.; Lin, J.; Li, W.; Li, J. The role of water in reducing WO3 film by hydrogen: Controlling the concentration of oxygen vacancies and improving the photoelectrochemical performance. J. Colloid Interface Sci. 2018, 512, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, M.; Shibuya, M.; Zhao, Z.G.; Liu, Z. Surface wetting behavior of a WO3 electrode under light-irradiated or potential-controlled conditions. J. Phys. Chem. C 2009, 113, 10642–10646. [Google Scholar] [CrossRef]
- Naseri, N.; Azimirad, R.; Akhavan, O.; Moshfegh, A.Z. The effect of nanocrystalline tungsten oxide concentration on surface properties of dip-coated hydrophilic WO3–SiO2 thin films. J. Phys. D Appl. Phys. 2007, 40, 2089–2095. [Google Scholar] [CrossRef]
- Ramana, C.V.; Battu, A.K.; Dubey, P.; Lopez, G.A. Phase-control-enabled enhancement in hydrophilicity and mechanical toughness in nanocrystalline tungsten oxide films for energy-related applications. ACS Appl. Nano Mater. 2020, 3, 3264–3274. [Google Scholar] [CrossRef]
- Raudoniene, J.; Laurikenas, A.; Kaba, M.M.; Sahin, G.; Morkan, A.U.; Brazinskiene, D.; Asadauskas, S.; Seidu, R.; Kareiva, A.; Garskaite, E. Textured WO3 and WO3:Mo films deposited from chemical solution on stainless steel. Thin Solid Film. 2018, 653, 179–187. [Google Scholar] [CrossRef]
- Top, I.; Binions, R.; Sol, C.; Papakonstantinou, I.; Holdynski, M.; Gaiaschi, S.; Abrahams, I. Improved thermochromic properties in bilayer films of VO2 with ZnO, SnO2 and WO3 coatings for energy efficient glazing. J. Mater. Chem. C 2018, 6, 12555. [Google Scholar] [CrossRef]
- Vardhan, R.V.; Kumar, S.; Mandal, S. A facile, low temperature spray pyrolysed tungsten oxide (WO3): An approach to antifouling coating by amalgamating scratch resistant and water repellent properties. Bull. Mater. Sci. 2020, 43, 281. [Google Scholar] [CrossRef]
- Vardhan, R.V.; Kumar, S.; Mandal, S. Fabrication of minimal capital-intensive scratch-resistant and hydrophobic tungsten oxide film on stainless steel through spray pyrolysis. Surf Interface Anal. 2022, 54, 510–523. [Google Scholar] [CrossRef]
- Vladuta, C.; Andronic, L.; Visa, M.; Duta, A. Ceramic interface properties evaluation based on contact angle measurement. Surf. Coat. Technol. 2008, 202, 2448–2452. [Google Scholar] [CrossRef]














| t (µm) | RMEA (Ω) | Rmembrane (Ω) | σ (mS cm−1) | |
|---|---|---|---|---|
| rNF | ~27.8 | 0.081 | 0.035 | 4.89 |
| rNF–WO-5 | 43 | 0.080 | 0.035 | 7.72 |
| rNF–WO-10 | 0.058 | 0.012 | 22.16 | |
| rNF–WO-15 | 0.088 | 0.042 | 6.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selim, A.; Szijjártó, G.P.; Románszki, L.; Tompos, A. Development of WO3–Nafion Based Membranes for Enabling Higher Water Retention at Low Humidity and Enhancing PEMFC Performance at Intermediate Temperature Operation. Polymers 2022, 14, 2492. https://doi.org/10.3390/polym14122492
Selim A, Szijjártó GP, Románszki L, Tompos A. Development of WO3–Nafion Based Membranes for Enabling Higher Water Retention at Low Humidity and Enhancing PEMFC Performance at Intermediate Temperature Operation. Polymers. 2022; 14(12):2492. https://doi.org/10.3390/polym14122492
Chicago/Turabian StyleSelim, Asmaa, Gábor Pál Szijjártó, Loránd Románszki, and András Tompos. 2022. "Development of WO3–Nafion Based Membranes for Enabling Higher Water Retention at Low Humidity and Enhancing PEMFC Performance at Intermediate Temperature Operation" Polymers 14, no. 12: 2492. https://doi.org/10.3390/polym14122492
APA StyleSelim, A., Szijjártó, G. P., Románszki, L., & Tompos, A. (2022). Development of WO3–Nafion Based Membranes for Enabling Higher Water Retention at Low Humidity and Enhancing PEMFC Performance at Intermediate Temperature Operation. Polymers, 14(12), 2492. https://doi.org/10.3390/polym14122492