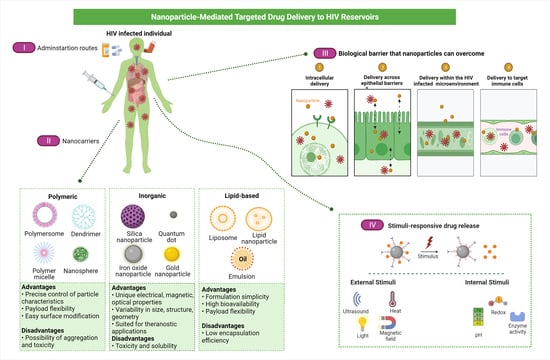
Novel Nanotechnology-Based Approaches for Targeting HIV Reservoirs
Abstract
:1. Introduction
1.1. Human Immunodeficiency Virus (HIV)
1.2. Replication Cycle
1.3. Epidemiology of HIV/AIDS
1.4. HIV Niches
1.4.1. Anatomical Reservoirs
Lymphoid Tissues
Liver
Gastrointestinal Tract
Lung
Kidneys
Central Nervous System (CNS)
Reproductive Tract
1.4.2. Cellular Reservoirs
CD4+ T Lymphocytes
Monocytes and Macrophage Lineage
Dendritic Cells
B Lymphocytes Cells
Natural Killer (NK) Cells
1.4.3. Molecular Reservoirs
1.5. Tackling the HIV: Challenges
1.5.1. Low Oral Bioavailability
1.5.2. Long-Term Drug Therapy
1.5.3. Toxicity
1.6. Nanopharmaceuticals; Novel Directions on HIV/AIDS Treatment Approaches
1.7. Nanoparticles Transport Approaches
1.7.1. Active Transport
Stimuli-Responsive Nanocarriers
Antibody Targeted Nanocarriers
Receptor-Mediated Endocytosis (RME)
The d-Mannose Receptor Targeting
1.7.2. Passive Targeting
Endocytosis
Phagocytosis
1.8. Factors Impacting the Functionalities of Nanocarrier Targeted Delivery
1.8.1. Particle Size
1.8.2. Particle Shape
1.8.3. Surface Charge
1.8.4. Surface Hydrophobicity
1.9. Liposomes-Based Delivery Systems for Anti-HIV Therapeutics
Liposomes-Based Delivery Systems of Ascorbic Acid to Increase the Bioavailability of ARTs
1.10. Nanotechnological Advantages for Effective Anti-HIV Therapy
2. Future Prospective
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalvi, B.R.; Siddiqui, E.A.; Syed, A.S.; Velhal, S.M.; Ahmad, A.; Bandivdekar, A.B.; Devarajan, P.V. Devarajan, P. Nevirapine Loaded Core Shell Gold Nanoparticles by Double Emulsion Solvent Evaporation: In Vitro and In Vivo Evaluation. Curr. Drug Deliv. 2016, 13, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Unaids.Org Global HIV & AIDS Statistics—2018 Fact Sheet|UNAIDS. Available online: http://www.unaids.org/en/resources/fact-sheet (accessed on 1 June 2022).
- Mahajan, K.; Rojekar, S.; Desai, D.; Kulkarni, S.; Vavia, P. Efavirenz Loaded Nanostructured Lipid Carriers for Efficient and Prolonged Viral Inhibition in HIV-Infected Macrophages. Pharm. Sci. 2020, 27, 418–432. [Google Scholar] [CrossRef]
- Mahajan, K.; Rojekar, S.; Desai, D.; Kulkarni, S.; Bapat, G.; Zinjarde, S.; Vavia, P. Layer-by-Layer Assembled Nanostructured Lipid Carriers for CD-44 Receptor–Based Targeting in HIV-Infected Macrophages for Efficient HIV-1 Inhibition. AAPS PharmSciTech 2021, 22, 171. [Google Scholar] [CrossRef] [PubMed]
- Rojekar, S.V.; Trimukhe, A.M.; Deshmukh, R.R.; Vavia, P.R. Novel pulsed oxygen plasma mediated surface hydrophılizatıon of ritonavır for the enhancement of wettability and solubility. J. Drug Deliv. Sci. Technol. 2021, 63, 102497. [Google Scholar] [CrossRef]
- Rojekar, S.; Pai, R.; Abadi, L.F.; Mahajan, K.; Prajapati, M.K.; Kulkarni, S.; Vavia, P. Dual loaded nanostructured lipid carrier of nano-selenium and Etravirine as a potential anti-HIV therapy. Int. J. Pharm. 2021, 607, 120986. [Google Scholar] [CrossRef] [PubMed]
- Rojekar, S.; Fotooh, L.; Pai, R.; Mahajan, K.; Kulkarni, S.; Vavia, P.R. Multi-organ Targeting of HIV-1 Viral Reservoirs with Etravirine Loaded Nanostructured Lipid Carrier: An In-vivo Proof of Concept. Eur. J. Pharm. Sci. 2021, 164, 105916. [Google Scholar] [CrossRef] [PubMed]
- Brechtl, J.R.; Breitbart, W.; Galietta, M.; Krivo, S.; Rosenfeld, B. The Use of Highly Active Antiretroviral Therapy (HAART) in Patients with Advanced HIV Infection: Impact on Medical, Palliative Care, and Quality of Life Outcomes. J. Pain Symptom Manag. 2001, 21, 41–51. [Google Scholar] [CrossRef]
- Simon, V.; Ho, D.D.; Karim, Q.A. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. Lancet 2006, 368, 489–504. [Google Scholar] [CrossRef] [Green Version]
- Sharp, P.; Hahn, B.H. Origins of HIV and the AIDS Pandemic. Cold Spring Harb. Perspect. Med. 2011, 1, a006841. [Google Scholar] [CrossRef] [Green Version]
- Preventive HIV Vaccines: Progress and Challenges. Available online: https://uspharmacist.com/article/preventive-hiv-vaccines-progress-and-challenges (accessed on 21 March 2021).
- HIV/AIDS. Available online: https://www.who.int/data/gho/data/themes/hiv-aids (accessed on 24 September 2020).
- Rojekar, S.; Vora, L.K.; Tekko, I.A.; Volpe-Zanutto, F.; McCarthy, H.O.; Vavia, P.R.; Donnelly, R.F. Etravirine-loaded dissolving microneedle arrays for long-acting delivery. Eur. J. Pharm. Biopharm. 2021, 165, 41–51. [Google Scholar] [CrossRef]
- WHO|World Health Organization. Available online: https://www.who.int/ (accessed on 4 November 2020).
- Statistics Overview|Statistics Center|HIV/AIDS|CDC. Available online: https://www.cdc.gov/hiv/statistics/overview/index.html (accessed on 4 November 2020).
- Kulkosky, J.; Bray, S. HAART-Persistent HIV-1 Latent Reservoirs: Their Origin, Mechanisms of Stability and Potential Strategies for Eradication. Curr. HIV Res. 2006, 4, 199–208. [Google Scholar] [CrossRef]
- Venzke, S.; Keppler, O.T. Role of macrophages in HIV infection and persistence. Expert Rev. Clin. Immunol. 2006, 2, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Avettand-Fènoël, V.; Hocqueloux, L.; Ghosn, J.; Cheret, A.; Frange, P.; Melard, A.; Viard, J.-P.; Rouzioux, C. Total HIV-1 DNA, a Marker of Viral Reservoir Dynamics with Clinical Implications. Clin. Microbiol. Rev. 2016, 29, 859–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haase, A.T. population Biology of Hiv-1 Infection: Viral and CD4+ T Cell Demographics and Dynamics in Lymphatic Tissues. Annu. Rev. Immunol. 1999, 17, 625–656. [Google Scholar] [CrossRef] [PubMed]
- Alex, A.; Paul, W.; Chacko, A.; Sharma, C.P. Enhanced delivery of lopinavir to the CNS using Compritol®-based solid lipid nanoparticles. Ther. Deliv. 2011, 2, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, H.; Herbst, H.; Niedobitek, G.; Foss, H.D.; Stein, H. Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells. Am. J. Pathol. 1992, 140, 15–22. [Google Scholar] [PubMed]
- Tenner-Rácz, K.; Rácz, P.; Schmidt, H.; Dietrich, M.; Kern, P.; Louie, A.; Gartner, S.; Popovic, M. Immunohistochemical, electron microscopic and in situ hybridization evidence for the involvement of lymphatics in the spread of HIV-1. AIDS 1988, 2, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.; Yukl, S.A. Tissue reservoirs of HIV. Curr. Opin. HIV AIDS 2016, 11, 362–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, C.C.; Onafuwa-Nuga, A.; McNamara, L.; Riddell, J.; Bixby, D.; Savona, M.R.; Collins, K.L. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat. Med. 2010, 16, 446–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yukl, S.A.; Sinclair, E.; Somsouk, M.; Hunt, P.W.; Epling, L.; Killian, M.; Girling, V.; Li, P.; Havlir, D.V.; Deeks, S.; et al. A comparison of methods for measuring rectal HIV levels suggests that HIV DNA resides in cells other than CD4+ T cells, including myeloid cells. AIDS 2014, 28, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, J.B.; Ellis, J.E.; Hair, G.A.; Kirshenbaum, A.S.; Metcalfe, D.D.; Yi, H.; Cardona, A.C.; Lindsay, M.K.; Ansari, A.A. Human tissue mast cells are an inducible reservoir of persistent HIV infection. Blood 2007, 109, 5293–5300. [Google Scholar] [CrossRef] [PubMed]
- Bourry, O.; Mannioui, A.; Sellier, P.; Roucairol, C.; Durand-Gasselin, L.; Dereuddre-Bosquet, N.; Benech, H.; Roques, P.; Le Grand, R. Effect of a short-term HAART on SIV load in macaque tissues is dependent on time of initiation and antiviral diffusion. Retrovirology 2010, 7, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, C.V.; Staskus, K.; Wietgrefe, S.W.; Rothenberger, M.; Reilly, C.; Chipman, J.G.; Beilman, G.J.; Khoruts, A.; Thorkelson, A.; Schmidt, T.E.; et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl. Acad. Sci. USA 2014, 111, 2307–2312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, M.; Poluektova, L.Y.; Kharbanda, K.K.; A Osna, N. Liver as a target of human immunodeficiency virus infection. World J. Gastroenterol. 2018, 24, 4728–4737. [Google Scholar] [CrossRef]
- Ahsan, M.H.; Gill, A.F.; Lackner, A.A.; Veazey, R.S. Acute and Chronic T Cell Dynamics in the Livers of Simian Immunodeficiency Virus-Infected Macaques. J. Virol. 2012, 86, 5244–5252. [Google Scholar] [CrossRef] [Green Version]
- Poles, M.A.; Elliott, J.; Taing, P.; Anton, P.A.; Chen, I.S.Y. A Preponderance of CCR5 + CXCR4 + Mononuclear Cells Enhances Gastrointestinal Mucosal Susceptibility to Human Immunodeficiency Virus Type 1 Infection. J. Virol. 2001, 75, 8390–8399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandtzaeg, P. Overview of the Mucosal Immune System. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 1989; Volume 146, pp. 13–25. [Google Scholar]
- A Jeffrey, A.; Israël-Biet, D.; Andrieu, J.M.; Even, P.; Venet, A. HIV isolation from pulmonary cells derived from bronchoalveolar lavage. Clin. Exp. Immunol. 1991, 84, 488–492. [Google Scholar]
- Crowe, S.M.; Westhorpe, C.L.V.; Mukhamedova, N.; Jaworowski, A.; Sviridov, D.; Bukrinsky, M. The macrophage: The intersection between HIV infection and atherosclerosis. J. Leukoc. Biol. 2009, 87, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Ross, M.D.; Bruggeman, L.A.; Hanss, B.; Sunamoto, M.; Marras, D.; Klotman, M.E.; Klotman, P.E. Podocan, a Novel Small Leucine-rich Repeat Protein Expressed in the Sclerotic Glomerular Lesion of Experimental HIV-associated Nephropathy. J. Biol. Chem. 2003, 278, 33248–33255. [Google Scholar] [CrossRef] [Green Version]
- Samikkannu, T.; Ranjith, D.; Rao, K.V.K.; Atluri, V.S.; Pimentel, E.; El-Hage, N.; Nair, M.P.N. HIV-1 gp120 and morphine induced oxidative stress: Role in cell cycle regulation. Front. Microbiol. 2015, 6, 614. [Google Scholar] [CrossRef] [PubMed]
- Navia, B.A.; Petito, C.K.; Gold, J.W.M.; Cho, E.-S.; Jordan, B.D.; Price, R.W. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: Clinical and neuropathological findings in 27 patients. Ann. Neurol. 1986, 19, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.K.; Rowe, T.; Curran, R.; Irving, W.L.; Beards, G.M.; Sontag, G.; Youle, M.; Moyle, G.; Pillay, D. Poor reduction of HIV-1 RNA titres in nucleoside reverse transcriptase inhibitor experienced patients treated with indinavir combination therapy. Sex. Transm. Infect. 1999, 75, 337–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajjar, A.M.; Lewis, P.F.; Endeshaw, Y.; Ndinya-Achola, J.; Kreiss, J.K.; Overbaugh, J. Efficient Isolation of Human Immunodeficiency Virus Type 1 RNA from Cervical Swabs. J. Clin. Microbiol. 1998, 36, 2349–2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liuzzi, G.; Chirianni, A.; Clementi, M.; Bagnarelli, P.; Valenza, A.; Cataldo, P.T.; Piazza, M. Analysis of HIV-1 load in blood, semen and saliva: Evidence for different viral compartments in a cross-sectional and longitudinal study. AIDS 1996, 10, F51–F56. [Google Scholar] [CrossRef]
- Gupta, P.; Mellors, J.; Kingsley, L.; Riddler, S.; Singh, M.K.; Schreiber, S.; Cronin, M.; Rinaldo, C.R. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J. Virol. 1997, 71, 6271–6275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Woodrow, K.A. Nanotechnology Approaches to Eradicating HIV Reservoirs. Eur. J. Pharm. Biopharm. 2019, 138, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Klatzmann, D.; Barré-Sinoussi, F.; Nugeyre, M.T.; Danguet, C.; Vilmer, E.; Griscelli, C.; Brun-Veziret, F.; Rouzioux, C.; Gluckman, J.C.; Chermann, J.-C.; et al. Selective Tropism of Lymphadenopathy Associated Virus (LAV) for Helper-Inducer T Lymphocytes. Science 1984, 225, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.D.; Rota, T.R.; Hirsch, M.S. Infection of monocyte/macrophages by human T lymphotropic virus type III. J. Clin. Investig. 1986, 77, 1712–1715. [Google Scholar] [CrossRef] [Green Version]
- Cavalu, S.; Bisboaca, S.; Mates, I.M.; Pasca, P.M.; Laslo, V.; Costea, T.; Fritea, L.; Vicas, S. Novel Formulation Based on Chitosan-Arabic Gum Nanoparticles Entrapping Propolis Extract Production, physico-chemical and structural characterization. Revista de Chimie 2018, 69, 12–3756. [Google Scholar] [CrossRef]
- Jindal, A.; Bachhav, S.; Devarajan, P.V. In situ hybrid nano drug delivery system (IHN-DDS) of antiretroviral drug for simultaneous targeting to multiple viral reservoirs: An in vivo proof of concept. Int. J. Pharm. 2017, 521, 196–203. [Google Scholar] [CrossRef]
- Lamers, S.L.; Salemi, M.; Galligan, D.C.; De Oliveira, T.; Fogel, G.B.; Granier, S.C.; Zhao, L.; Brown, J.N.; Morris, A.; Masliah, E.; et al. Extensive HIV-1 Intra-Host Recombination Is Common in Tissues with Abnormal Histopathology. PLoS ONE 2009, 4, e5065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsden, M.D.; Zack, J.A. Eradication of HIV: Current challenges and new directions. J. Antimicrob. Chemother. 2008, 63, 7–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentin, A.; Pavlakis, G.N. Natural killer cells are persistently infected and resistant to direct killing by HIV-1. Anticancer Res. 2003, 23, 2071–2075. [Google Scholar]
- Geijtenbeek, T.B.; Kwon, D.S.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.C.; Middel, J.; Cornelissen, I.L.; Nottet, H.S.; KewalRamani, V.N.; Littman, D.R.; et al. DC-SIGN, a Dendritic Cell–Specific HIV-1-Binding Protein that Enhances trans-Infection of T Cells. Cell 2000, 100, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Turville, S.; Cameron, P.U.; Handley, A.; Lin, G.; Pöhlmann, S.; Doms, R.W.; Cunningham, A. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 2002, 3, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Olinger, G.G.; Saifuddin, M.; Spear, G.T. CD4-Negative Cells Bind Human Immunodeficiency Virus Type 1 and Efficiently Transfer Virus to T Cells. J. Virol. 2000, 74, 8550–8557. [Google Scholar] [CrossRef] [Green Version]
- Valentin, A.; Rosati, M.; Patenaude, D.J.; Hatzakis, A.; Kostrikis, L.G.; Lazanas, M.; Wyvill, K.M.; Yarchoan, R.; Pavlakis, G.N. Persistent HIV-1 infection of natural killer cells in patients receiving highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2002, 99, 7015–7020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisele, E.; Siliciano, R.F. Redefining the Viral Reservoirs that Prevent HIV-1 Eradication. Immunity 2012, 37, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Pierson, T.; McArthur, J.; Siliciano, R.F. Reservoirs for HIV-1: Mechanisms for Viral Persistence in the Presence of Antiviral Immune Responses and Antiretroviral Therapy. Annu. Rev. Immunol. 2000, 18, 665–708. [Google Scholar] [CrossRef]
- Verweel, G.; van Rossum, A.M.C.; Hartwig, N.G.; Wolfs, T.F.W.; Scherpbier, H.J.; de Groot, R. Treatment with Highly Active Antiretroviral Therapy in Human Immunodeficiency Virus Type 1-Infected Children Is Associated with a Sustained Effect on Growth. Pediatrics 2002, 109, e25. [Google Scholar] [CrossRef] [Green Version]
- Kumar, L.; Verma, S.; Prasad, D.N.; Bhardwaj, A.; Vaidya, B.; Jain, A.K. Nanotechnology: A magic bullet for HIV AIDS treatment. Artif. Cells Nanomed. Biotechnol. 2014, 43, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Esté, J.A.; Cihlar, T. Current status and challenges of antiretroviral research and therapy. Antivir. Res. 2010, 85, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, N.; Blas-García, A.; Esplugues, J.V. Mitochondrial interference by anti-HIV drugs: Mechanisms beyond Pol-γ inhibition. Trends Pharmacol. Sci. 2011, 32, 715–725. [Google Scholar] [CrossRef]
- Sosnik, A.; Chiappetta, D.A.; Carcaboso, M. Drug delivery systems in HIV pharmacotherapy: What has been done and the challenges standing ahead. J. Control. Release 2009, 138, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, L.; Zhang, X.; Zhang, J.; Shang, H.; Liang, G. Neuropilin-1, a myeloid cell-specific protein, is an inhibitor of HIV-1 infectivity. Proc. Natl. Acad. Sci. USA 2022, 119, e2114884119. [Google Scholar] [CrossRef]
- Kedzierska, K. The Role of Monocytes and Macrophages in the Pathogenesis of HIV-1 Infection. Curr. Med. Chem. 2002, 9, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- McGee, B.; Smith, N.; Aweeka, F. HIV Pharmacology: Barriers to the Eradication of HIV from the CNS. HIV Clin. Trials 2006, 7, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Vyas, T.K.; Shah, L.; Amiji, M.M. Nanoparticulate drug carriers for delivery of HIV/AIDS therapy to viral reservoir sites. Expert Opin. Drug Deliv. 2006, 3, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Asthana, G.S.; Asthana, A.; Kohli, D.V.; Vyas, S.P. Mannosylated Chitosan Nanoparticles for Delivery of Antisense Oligonucleotides for Macrophage Targeting. BioMed Res. Int. 2014, 2014, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, L.; Pooyan, S.; Hu, P.; Leibowitz, M.J.; Stein, S.; Sinko, P.J. Peritoneal Macrophage Uptake, Pharmacokinetics and Biodistribution of Macrophage-Targeted PEG-fMLF (N-Formyl-Methionyl-Leucyl-Phenylalanine) Nanocarriers for Improving HIV Drug Delivery. Pharm. Res. 2007, 24, 2110–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowacek, A.; E Gendelman, H. NanoART, neuroAIDS and CNS drug delivery. Nanomedicine 2009, 4, 557–574. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Sravanam, S.; Sillman, B.; Waight, E.; Makarov, E.; Mathews, S.; Poluektova, L.Y.; Gorantla, S.; Gendelman, H.E.; Dash, P.K. Recovery of Latent HIV-1 from Brain Tissue by Adoptive Cell Transfer in Virally Suppressed Humanized Mice. J. Neuroimmune Pharmacol. 2021, 16, 796–805. [Google Scholar] [CrossRef]
- Lin, J.H.; Chen, I.W.; Vastag, K.J.; Ostovic, D. PH-Dependent Oral Absorption of L-735,524, a Potent HIV Protease Inhibitor, in Rats and Dogs. Drug Metab. Dispos. 1995, 23, 730–735. [Google Scholar] [PubMed]
- Kwei, G.Y.; Novak, L.B.; Hettrick, L.A.; Reiss, E.R.; Ostovic, D.; Loper, A.E.; Lui, C.Y.; Higgins, R.J.; Chen, I.; Lin, J.H. Regiospecific Intestinal Absorption of the HIV Protease Inhibitor L-735,524 in Beagle Dogs. Pharm. Res. 1995, 12, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Blankson, J.N.; Persaud, D.; Siliciano, R.F. The Challenge of Viral Reservoirs in HIV-1 Infection. Annu. Rev. Med. 2002, 53, 557–593. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, G. Heart and HAART: Two sides of the coin for HIV-associated cardiology issues. World J. Cardiol. 2010, 2, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Highly Active Antiretroviral Therapy (HAART)—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554533/ (accessed on 21 August 2020).
- Bocedi, A.; Notaril, S.; Narciso, P.; Bolli, A.; Fasano, M.; Ascenzi, P. Binding of Anti-HIV Drugs to Human Serum Albumin. IUBMB Life 2004, 56, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, D.; Damiri, F.; Rojekar, S.; Zehravi, M.; Ramproshad, S.; Dhoke, D.; Musale, S.; Mulani, A.A.; Modak, P.; Paradhi, R.; et al. Recent Advancements in Microneedle Technology for Multifaceted Biomedical Applications. Pharmaceutics 2022, 14, 1097. [Google Scholar] [CrossRef]
- Greene, J.N.; Poblete, S.J.; Krieff, D. New directions in antimicrobial therapy. Chest Surg. Clin. N. Am. 1999, 9, 39–61. [Google Scholar] [PubMed]
- Pennings, P.S. HIV drug resistance: Problems and perspectives. Infect. Dis. Rep. 2013, 5, e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rugemalila, J.; Kamori, D.; Maokola, W.; Mizinduko, M.; Barabona, G.; Masoud, S.; Mlunde, L.B.; Mutagonda, R.F.; Ruhago, G.; Mushi, J.; et al. Acquired HIV drug resistance among children and adults receiving antiretroviral therapy in Tanzania: A national representative survey protocol. BMJ Open 2021, 11, e054021. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, M.; Mohri, H.; Mehandru, S.; Shet, A.; Berry, L.; Kalyanaraman, R.; Kim, A.; Chung, C.; Jean-Pierre, P.; Horowitz, A.; et al. Infection with multidrug resistant, dual-tropic HIV-1 and rapid progression to AIDS: A case report. Lancet 2005, 365, 1031–1038. [Google Scholar] [CrossRef]
- Rao, K.S.; Ghorpade, A.; Labhasetwar, V. Targeting anti-HIV drugs to the CNS. Expert Opin. Drug Deliv. 2009, 6, 771–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarma, A.; Das, M.K. Nose to brain delivery of antiretroviral drugs in the treatment of neuroAIDS. Mol. Biomed. 2020, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, B.; Morgan, J.; Showalter, L.; Horng, K.R.; Dandekar, S.; Herrera, C.; LiWang, P.; Kaplan, D.L. Pharmaceutical Approaches to HIV Treatment and Prevention. Adv. Ther. 2018, 1, 1800054. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Wang, C.; Patton, C.; Murray, M.; Punekar, Y.; de Ruiter, A.; Steinhart, C. A Review of Long-Term Toxicity of Antiretroviral Treatment Regimens and Implications for an Aging Population. Infect. Dis. Ther. 2018, 7, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Trickey, A.; May, M.T.; Vehreschild, J.-J.; Obel, N.; Gill, M.J.; Crane, H.M.; Boesecke, C.; Patterson, S.; Grabar, S.; Cazanave, C.; et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: A collaborative analysis of cohort studies. Lancet HIV 2017, 4, e349–e356. [Google Scholar] [CrossRef] [Green Version]
- Prokofjeva, M.M.; Kochetkov, S.N.; Prassolov, V.S. Therapy of HIV Infection: Current Approaches and Prospects. Acta Naturae 2016, 8, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Ho, D.D. Therapy of HIV infections: Problems and prospects. Bull. N. Y. Acad. Med. 1996, 73, 37–45. [Google Scholar]
- Cihlar, T.; Fordyce, M. Current status and prospects of HIV treatment. Curr. Opin. Virol. 2016, 18, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Crowe, S.M.; McGrath, M.S.; Elbeik, T.; Kirihara, J.; Mills, J. Comparative assessment of antiretrovirals in human monocyte-macrophages and lymphoid cell lines acutely and chronically infected with the human immunodeficiency virus. J. Med Virol. 1989, 29, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.H.; Hearps, A.C.; Martin, G.E.; Williams, K.C.; Crowe, S.M. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. AIDS 2014, 28, 2175–2187. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Rahman, H.; Zehravi, M.; Awaji, A.A.; Nasrullah, M.Z.; Gad, H.A.; Bani-Fwaz, M.Z.; Varma, R.S.; Germoush, M.O.; Al-Malky, H.S.; et al. MXene (Ti3C2Tx)-Embedded Nanocomposite Hydrogels for Biomedical Applications: A Review. Materials 2022, 15, 1666. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Regional Office for the Eastern Mediterranean Eastern Mediterranean Health Journal. Available online: http://www.emro.who.int/asd/about/hiv-situation-region.html (accessed on 19 June 2022).
- World Health Organization. Antiretroviral Therapy of HIV Infection in Infants and Children: Towards Universal Access: Recommendations for a Public Health Approach—2010 Revision; World Health Organization: Geneva, Switzerland, 2010; ISBN 978-92-4-159980-1. [Google Scholar]
- Carr, A. Toxicity of antiretroviral therapy and implications for drug development. Nat. Rev. Drug Discov. 2003, 2, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Sornsuwan, K.; Thongkhum, W.; Pamonsupornwichit, T.; Carraway, T.S.; Soponpong, S.; Sakkhachornphop, S.; Tayapiwatana, C.; Yasamut, U. Performance of Affinity-Improved DARPin Targeting HIV Capsid Domain in Interference of Viral Progeny Production. Biomolecules 2021, 11, 1437. [Google Scholar] [CrossRef]
- Kakuda, T.N. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin. Ther. 2000, 22, 685–708. [Google Scholar] [CrossRef]
- Shikuma, C.M.; Shiramizu, B. Mitochondrial toxicity associated with nucleoside reverse transcriptase inhibitor therapy. Curr. Infect. Dis. Rep. 2001, 3, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Baril, J.-G.; Junod, P.; LeBlanc, R.; Dion, H.; Therrien, R.; Laplante, F.; Falutz, J.; Côté, P.; Hébert, M.-N.; Lalonde, R.; et al. HIV-associated Lipodystrophy Syndrome: A Review of Clinical Aspects. Can. J. Infect. Dis. Med Microbiol. 2005, 16, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Guaraldi, G.; Stentarelli, C.; Zona, S.; Santoro, A. HIV-Associated Lipodystrophy: Impact of Antiretroviral Therapy. Drugs 2013, 73, 1431–1450. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, Y.; Yoshino, H. Lymphatic targeting with nanoparticulate system. Adv. Drug Deliv. Rev. 2001, 47, 55–64. [Google Scholar] [CrossRef]
- Cavalu, S.; Banica., F.; Gruian, C.; Vanea, E.; Goller, G.; Simon, V. Microscopic and spectroscopic investigation of bioactive glasses for antibiotic controlled release. J. Mol. Struct. 2013, 1040, 47–52. [Google Scholar] [CrossRef]
- Lee, E.S.; Na, K.; Bae, Y.H. Super pH-Sensitive Multifunctional Polymeric Micelle. Nano Lett. 2005, 5, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Kono, K. Thermosensitive polymer-modified liposomes. Adv. Drug Deliv. Rev. 2001, 53, 307–319. [Google Scholar] [CrossRef]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia Enables Tumor-Specific Nanoparticle Delivery: Effect of Particle Size 1. Cancer Res. 2000, 60, 4440–4445. [Google Scholar] [PubMed]
- Chen, Q.; Krol, A.; Wright, A.; Nedham, D.; Dewhirst, M.W.; Yuan, F. Tumor microvascular permeability is a key determinant for antivascular effects of doxorubicin encapsulated in a temperature sensitive liposome. Int. J. Hyperth. 2008, 24, 475–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.; Yang, J.; Choi, J.; Suh, J.-S.; Huh, Y.-M.; Haam, S. Synthesis of gold nanorod-embedded polymeric nanoparticles by a nanoprecipitation method for use as photothermal agents. Nanotechnology 2009, 20, 365602. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ban, H.-S.; Kim, S.-S.; Wu, H.; Pearson, T.; Greiner, D.L.; Laouar, A.; Yao, J.; Haridas, V.; Habiro, K.; et al. T Cell-Specific siRNA Delivery Suppresses HIV-1 Infection in Humanized Mice. Cell 2008, 134, 577–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagné, J.-F.; Désormeaux, A.; Perron, S.; Tremblay, M.J.; Bergeron, M.G. Targeted delivery of indinavir to HIV-1 primary reservoirs with immunoliposomes. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2001, 1558, 198–210. [Google Scholar] [CrossRef] [Green Version]
- Delcroix, M.; Riley, L.W. Cell-Penetrating Peptides for Antiviral Drug Development. Pharmaceuticals 2010, 3, 448–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azad, A.K.; Rajaram, M.V.S.; Schlesinger, L.S. Exploitation of the Macrophage Mannose Receptor (CD206) in Infectious Disease Diagnostics and Therapeutics. J. Cytol. Mol. Biol. 2014, 1, 1000003. [Google Scholar] [CrossRef]
- Fraser, I.P.; Ezekowitz, R.A.B. Mannose Receptor and Phagocytosis. Adv. Cell. Mol. Biol. Membr. Organelles 1999, 5, 87–101. [Google Scholar]
- Bañó, M.; Morén, C.; Barroso, S.; Juárez, D.L.; Guitart-Mampel, M.; González-Casacuberta, I.; Canto-Santos, J.; Lozano, E.; León, A.; Pedrol, E.; et al. Mitochondrial Toxicogenomics for Antiretroviral Management: HIV Post-exposure Prophylaxis in Uninfected Patients. Front. Genet. 2020, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.; Miller, J.; Law, M.; Cooper, D.A. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: Contribution to protease inhibitor-related lipodystrophy syndrome. AIDS 2000, 14, F25–F32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Song, S.Y.; Lee, S.G.; Choi, S.; Lee, Y.I.; Choi, J.Y.; Lee, J.H. Treatment of Human Immunodeficiency Virus-Associated Facial Lipoatrophy with Hyaluronic Acid Filler Mixed With Micronized Cross-Linked Acellular Dermal Matrix. J. Korean Med Sci. 2022, 37, e37. [Google Scholar] [CrossRef]
- Kuo, H.-H.; Lichterfeld, M. Recent progress in understanding HIV reservoirs. Curr. Opin. HIV AIDS 2018, 13, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Z.; Ander, B.P.; Xu, H.; Shen, Y.; Kaur, P.; Deng, W.; Sharp, F.R. Blood-brain barrier breakdown and repair by Src after thrombin-induced injury. Ann. Neurol. 2009, 67, 526–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics 2018, 10, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Heijden, J.W.; Oerlemans, R.; Dijkmans, B.A.C.; Qi, H.; Van Der Laken, C.J.; Lems, W.F.; Jackman, A.L.; Kraan, M.C.; Tak, P.P.; Ratnam, M.; et al. Folate receptor β as a potential delivery route for novel folate antagonists to macrophages in the synovial tissue of rheumatoid arthritis patients. Arthritis Care Res. 2008, 60, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Puig-Kröger, A.; Sierra-Filardi, E.; Domínguez-Soto, A.; Samaniego, R.; Corcuera, M.T.; Gómez-Aguado, F.; Ratnam, M.; Sánchez-Mateos, P.; Corbí, A.L. Folate Receptor β Is Expressed by Tumor-Associated Macrophages and Constitutes a Marker for M2 Anti-inflammatory/Regulatory Macrophages. Cancer Res. 2009, 69, 9395–9403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzehoval, E.; Segal, S.; Stabinsky, Y.; Fridkin, M.; Spirer, Z.; Feldman, M. Tuftsin (an Ig-associated tetrapeptide) triggers the immunogenic function of macrophages: Implications for activation of programmed cells. Proc. Natl. Acad. Sci. USA 1978, 75, 3400–3404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tempone, A.G.; Pimenta, D.C.; Lebrun, I.; Sartorelli, P.; Taniwaki, N.N.; de Andrade, H.F., Jr.; Antoniazzi, M.M.; Jared, C. Antileishmanial and antitrypanosomal activity of bufadienolides isolated from the toad Rhinella jimi parotoid macrogland secretion. Toxicon 2008, 52, 13–21. [Google Scholar] [CrossRef]
- Banerjee, D.; Liu, A.P.; Voss, N.R.; Schmid, S.L.; Finn, M.G. Multivalent Display and Receptor-Mediated Endocytosis of Transferrin on Virus-Like Particles. ChemBioChem 2010, 11, 1273–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilliams, M.; Bruhns, P.; Saeys, Y.; Hammad, H.; Lambrecht, B.N. The function of Fcγ receptors in dendritic cells and macrophages. Nat. Rev. Immunol. 2014, 14, 94–108. [Google Scholar] [CrossRef] [PubMed]
- E Taylor, M.; Bezouska, K.; Drickamer, K. Contribution to ligand binding by multiple carbohydrate-recognition domains in the macrophage mannose receptor. J. Biol. Chem. 1992, 267, 1719–1726. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like Receptor and RIG-1-like Receptor Signaling. Ann. N. Y. Acad. Sci. 2008, 1143, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Campagne, M.V.L.; Wiesmann, C.; Brown, E.J. Macrophage complement receptors and pathogen clearance. Cell. Microbiol. 2007, 9, 2095–2102. [Google Scholar] [CrossRef]
- Dubey, V.; Nahar, M.; Mishra, D.; Mishra, P.; Jain, N.K. Surface structured liposomes for site specific delivery of an antiviral agent-indinavir. J. Drug Target. 2010, 19, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Asthana, A.; Agashe, H.B.; Agrawal, G.P.; Jain, N.K. Stavudine-loaded mannosylated liposomes: In-vitro anti-HIV-I activity, tissue distribution and pharmacokinetics. J. Pharm. Pharmacol. 2006, 58, 605–616. [Google Scholar] [CrossRef]
- Garg, M.; Dutta, T.; Jain, N.K. Reduced hepatic toxicity, enhanced cellular uptake and altered pharmacokinetics of stavudine loaded galactosylated liposomes. Eur. J. Pharm. Biopharm. 2007, 67, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Heiati, H.; Tawashi, R.; Phillips, N.C. Solid lipid nanoparticles as drug carriers: II. Plasma stability and biodistribution of solid lipid nanoparticles containing the lipophilic prodrug 3′-azido-3′-deoxythymidine palmitate in mice. Int. J. Pharm. 1998, 174, 71–80. [Google Scholar] [CrossRef]
- Kaur, C.D.; Nahar, M.; Jain, N.K. Lymphatic targeting of zidovudine using surface-engineered liposomes. J. Drug Target. 2008, 16, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Shahiwala, A.; Amiji, M.M. Nanotechnology-based delivery systems in HIV/AIDS therapy. Futur. HIV Ther. 2007, 1, 49–59. [Google Scholar] [CrossRef]
- Gaillard, P.J.; Visser, C.C.; de Boer, M.; Appeldoorn, C.C.M.; Rip, J. Blood-to-Brain Drug Delivery Using Nanocarriers. AAPS Adv. Pharm. Sci. Ser. 2014, 10, 433–454. [Google Scholar] [CrossRef]
- Jain, S.K.; Gupta, Y.; Jain, A.; Saxena, A.R.; Khare, P.; Jain, A. Mannosylated gelatin nanoparticles bearing an anti-HIV drug didanosine for site-specific delivery. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Jain, N.K. Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2007, 1770, 681–686. [Google Scholar] [CrossRef]
- Patel, B.K.; Parikh, R.H.; Patel, N. Targeted delivery of mannosylated-PLGA nanoparticles of antiretroviral drug to brain. Int. J. Nanomed. 2018, 13, 97–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, A.; Jain, S.; Tiwary, A. Mannan-coated gelatin nanoparticles for sustained and targeted delivery of didanosine: In vitro and in vivo evaluation. Acta Pharm. 2008, 58, 61–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, K.S.; Reddy, M.K.; Horning, J.L.; Labhasetwar, V. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials 2008, 29, 4429–4438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, Y.-C.; Wang, L.-J. Transferrin-grafted catanionic solid lipid nanoparticles for targeting delivery of saquinavir to the brain. J. Taiwan Inst. Chem. Eng. 2014, 45, 755–763. [Google Scholar] [CrossRef]
- Lavigne, C.; Guedj, A.-S.; Kell, A.J.; Barnes, M.; Stals, S.; Gonçalves, D.; Girard, D. Preparation, characterization, and safety evaluation of poly (lactide-co-glycolide) nanoparticles for protein delivery into macrophages. Int. J. Nanomed. 2015, 10, 5965–5979. [Google Scholar] [CrossRef] [Green Version]
- Shegokar, R. Preparation, Characterization and Cell Based Delivery of Stavudine Surface Modified Lipid Nanoparticles. J. Nanomed. Biother. Discov. 2012, 2. [Google Scholar] [CrossRef]
- Yu, S.S.; Lau, C.M.; Barham, W.J.; Onishko, H.M.; Nelson, C.E.; Li, H.; Smith, C.A.; Yull, F.E.; Duvall, C.L.; Giorgio, T.D. Macrophage-Specific RNA Interference Targeting via “Click”, Mannosylated Polymeric Micelles. Mol. Pharm. 2013, 10, 975–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Prakoso, A.T.; Basri, H.; van der Heide, E. Computational Contact Pressure Prediction of CoCrMo, SS 316L and Ti6Al4V Femoral Head against UHMWPE Acetabular Cup under Gait Cycle. J. Funct. Biomater. 2022, 13, 64. [Google Scholar] [CrossRef]
- Shibata, A.; McMullen, E.; Pham, A.; Belshan, M.; Sanford, B.; Zhou, Y.; Goede, M.; Date, A.; Destache, C.J. Polymeric Nanoparticles Containing Combination Antiretroviral Drugs for HIV Type 1 Treatment. AIDS Res. Hum. Retroviruses 2013, 29, 746–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Zhou, Q.; Li, Y.; Garner, L.V.; Watkins, S.P.; Carter, L.J.; Smoot, J.; Gregg, A.C.; Daniels, A.D.; Jervey, S.; et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent. Sci. 2020, 6, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Malik, T.; Chauhan, G.; Rath, G.; Kesarkar, R.N.; Chowdhary, A.S.; Goyal, A.K. Efaverinz and nano-gold-loaded mannosylated niosomes: A host cell-targeted topical HIV-1 prophylaxis via thermogel system. Artif. Cells Nanomed. Biotechnol. 2017, 46, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Vieira, A.C.; Magalhães, J.; Rocha, S.; Cardoso, M.S.; Santos, S.G.; Borges, M.; Pinheiro, M.; Reis, S. Targeted macrophages delivery of rifampicin-loaded lipid nanoparticles to improve tuberculosis treatment. Nanomedicine 2017, 12, 2721–2736. [Google Scholar] [CrossRef]
- Makabi-Panzu, B.; Lessard, C.; Beauchamp, D.; Désormeaux, A.; Poulin, L.; Tremblay, M.; Bergeron, M.G. Uptake and Binding of Liposomal 2’,3’-Dideoxycytidine by RAW 264.7 Cells: A Three-Step Process. J. Acquir. Immune Defic. Syndr. Hum. Retrovirology 1995, 8, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Feng, S.-S. Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Agarwal, A.; Majumder, S.; Lariya, N.; Khaya, A.; Agrawal, H.; Majumdar, S.; Agrawal, G.P. Mannosylated solid lipid nanoparticles as vectors for site-specific delivery of an anti-cancer drug. J. Control. Release 2010, 148, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Edagwa, B.J.; Zhou, T.; McMillan, J.M.; Liu, X.-M.; E Gendelman, H. Development of HIV Reservoir Targeted Long Acting Nanoformulated Antiretroviral Therapies. Curr. Med. Chem. 2014, 21, 4186–4198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trimukhe, A.; Rojekar, S.; Vavia, P.R.; Deshmukh, R. Pulsed plasma surface modified omeprazole microparticles for delayed release application. J. Drug Deliv. Sci. Technol. 2021, 66, 102905. [Google Scholar] [CrossRef]
- Nie, S. Understanding and Overcoming Major Barriers in Cancer Nanomedicine. Opsonization Phagocytosis 2010, 5, 523–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litzinger, D.C.; Buiting, A.M.; van Rooijen, N.; Huang, L. Effect of liposome size on the circulation time and intraorgan distribution of amphipathic poly(ethylene glycol)-containing liposomes. Biochim. Biophys. Acta (BBA)—Biomembr. 1994, 1190, 99–107. [Google Scholar] [CrossRef]
- Saste, V.S.; Kale, S.S.; Sapate, M.K.; Baviskar, D.T. Modern Aspects for Antiretroviral Treatment. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 18–24. [Google Scholar]
- Raina, H.; Kaur, S.; Jindal, A.B. Development of efavirenz loaded solid lipid nanoparticles: Risk assessment, quality-by-design (QbD) based optimisation and physicochemical characterisation. J. Drug Deliv. Sci. Technol. 2017, 39, 180–191. [Google Scholar] [CrossRef]
- Alukda, D.; Sturgis, T.; Youan, B.C. Formulation of tenofovir-loaded functionalized solid lipid nanoparticles intended for HIV prevention. J. Pharm. Sci. 2011, 100, 3345–3356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hari, B.V.; Narayanan, N.; Dhevendaran, K.; Ramyadevi, D. Engineered nanoparticles of Efavirenz using methacrylate co-polymer (Eudragit-E100) and its biological effects in-vivo. Mater. Sci. Eng. C 2016, 67, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Iannazzo, D.; Pistone, A.; Romeo, R.; Giofrè, S.V. Nanotechnology Approaches for Antiretroviral Drugs Delivery. J. AIDS HIV Infect. 2015, 1, 1–13. [Google Scholar] [CrossRef]
- Kotta, S.; Khan, A.W.; Ansari, S.H.; Sharma, R.K.; Ali, J. Anti HIV nanoemulsion formulation: Optimization and in vitro–in vivo evaluation. Int. J. Pharm. 2014, 462, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Beg, S.; Kumar, R.; Katare, O.; Singh, B. Nanostructured lipidic carriers of lopinavir for effective management of HIV-associated neurocognitive disorder. J. Drug Deliv. Sci. Technol. 2019, 53, 101220. [Google Scholar] [CrossRef]
- Chakraborty, T.; Das, M.K.; Dutta, L.; Mukherjee, B.; Das, S.; Sarma, A. Successful Delivery of Zidovudine-Loaded Docosanol Nanostructured Lipid Carriers (Docosanol NLCs) into Rat Brain. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 245–276. [Google Scholar]
- Dusserre, N.; Lessard, C.; Paquette, N.; Perron, S.; Poulin, L.; Tremblay, M.; Beauchamp, D.; Désormeaux, A.; Bergeron, M.G. Encapsulation of foscarnet in liposomes modifies drug intracellular accumulation, in vitro anti-HIV-1 activity, tissue distribution, and pharmacokinetics. AIDS 1995, 9, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.A. Pharmacytes: An Ideal Vehicle for Targeted Drug Delivery. J. Nanosci. Nanotechnol. 2006, 6, 2769–2775. [Google Scholar] [CrossRef] [PubMed]
- Houacine, C.; Adams, D.; Singh, K. Impact of liquid lipid on development and stability of trimyristin nanostructured lipid carriers for oral delivery of resveratrol. J. Mol. Liq. 2020, 316, 113734. [Google Scholar] [CrossRef]
- Désormeaux, A.; Harvie, P.; Perron, S.; Makabi-Panzu, B.; Beauchamp, D.; Tremblay, M.; Poulin, L.; Bergeron, M.G. Antiviral efficacy, intracellular uptake and pharmacokinetics of free and liposome-encapsulated 2’,3’-dideoxyinosine. AIDS 1994, 8, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Purvin, S.; Vuddanda, P.R.; Singh, S.K.; Jain, A. Pharmacokinetic and Tissue Distribution Study of Solid Lipid Nanoparticles of Zidovudine in Rats. J. Nanotechnol. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pharmacokinetic and Tissue Distribution of Zidovudine in Rats Following Intravenous Administration of Zidovudine Myristate Loaded Liposomes. Available online: https://www.researchgate.net/publication/7448025_Pharmacokinetic_and_tissue_distribution_of_zidovudine_in_rats_following_intravenous_administration_of_zidovudine_myristate_loaded_liposomes (accessed on 22 March 2021).
- Destache, C.J.; Belgum, T.; Goede, M.; Shibata, A.; Belshan, M.A. Antiretroviral release from poly (DL-lactide-co-glycolide) nanoparticles in mice. J. Antimicrob. Chemother. 2010, 65, 2183–2187. [Google Scholar] [CrossRef] [Green Version]
- Publications. Available online: https://universe.bits-pilani.ac.in/Hyderabad/punnaraoravi/Publications (accessed on 22 March 2021).
- Davidson, I.; Beardsell, H.; Smith, B.; Mandalia, S.; Bower, M.; Gazzard, B.; Nelson, M.; Stebbing, J. The frequency and reasons for antiretroviral switching with specific antiretroviral associations: The SWITCH study. Antivir. Res. 2010, 86, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.K.; Mishra, S.; Bajpai, M.; Mishra, A. Enhanced Oral Bioavailability of Efavirenz by Solid Lipid Nanoparticles: In Vitro Drug Release and Pharmacokinetics Studies. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, N.; Zastre, J.; Wong, H.-L.; Wu, X.Y.; Bendayan, R. Solid Lipid Nanoparticles Enhance the Delivery of the HIV Protease Inhibitor, Atazanavir, by a Human Brain Endothelial Cell Line. Pharm. Res. 2008, 25, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Lakshmi, Y.S.; Bhaskar, C.; Golla, K.; Kondapi, A.K. Improved Safety, Bioavailability and Pharmacokinetics of Zidovudine through Lactoferrin Nanoparticles during Oral Administration in Rats. PLoS ONE 2015, 10, e0140399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, H.; Destache, C.; Morehead, J.R.; Mosley, R.L.; Boska, M.D.; Kingsley, J.; Gorantla, S.; Poluektova, L.; Nelson, J.A.; Chaubal, M.; et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood 2006, 108, 2827–2835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, P.S.; Read, S.W. Nanotechnology and HIV: Potential applications for treatment and prevention. WIREs Nanomed. Nanobiotechnology 2010, 2, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; McMillan, J.E.M.; Gendelman, H.E. Nanomedicines for Nervous System Diseases. In Handbook of Neurotoxicity; Springer: New York, NY, USA, 2014; Volume 3, pp. 2125–2156. ISBN 9781461458364. [Google Scholar]
- Shegokar, R.; Singh, K.K. Surface modified nevirapine nanosuspensions for viral reservoir targeting: In vitro and in vivo evaluation. Int. J. Pharm. 2011, 421, 341–352. [Google Scholar] [CrossRef]
- Dou, H.; Grotepas, C.B.; McMillan, J.M.; Destache, C.; Chaubal, M.; Werling, J.; Kipp, J.; Rabinow, B.; Gendelman, H.E. Macrophage Delivery of Nanoformulated Antiretroviral Drug to the Brain in a Murine Model of NeuroAIDS. J. Immunol. 2009, 183, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Van Gyseghem, E.; Pendela, M.; Baert, L.; Rosier, J.; Klooster, G.V.; De Man, H.; Bouche, M.-P.; Schueller, L.; Van Remoortere, P.; Wigerinck, P.; et al. Powder for reconstitution of the anti-HIV-1 drug TMC278—Formulation development, stability and animal studies. Eur. J. Pharm. Biopharm. 2008, 70, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release 2007, 121, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devarajan, P.V.; Jindal, A.B.; Patil, R.R.; Mulla, F.; Gaikwad, R.V.; Samad, A. Particle Shape: A New Design Parameter for Passive Targeting in Splenotropic Drug Delivery. J. Pharm. Sci. 2010, 99, 2576–2581. [Google Scholar] [CrossRef]
- Mishra, D.; Jindal, A.B. Lipid Based Nanocarriers for Delivery of Anti-HIV Drugs: A Mini Review. Nanosci. Nanotechnol.—Asia 2018, 8, 172–185. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza, A.A.; Joshi, V.M.; Devarajan, P.V. Hepatic Targeting: Physiological Basis and Design Strategy; Springer: Cham, Switzerland, 2015; pp. 197–238. [Google Scholar]
- Devarajan, P.V.; Dawre, S.M.; Dutta, R. Infectious Diseases: Need for Targeted Drug Delivery; Springer: Cham, Switzerland, 2015; pp. 113–148. [Google Scholar]
- Jiménez, J.L.; Clemente, M.I.; Weber, N.D.; Sanchez, J.; Ortega, P.; De La Mata, F.J.; Gómez, R.; García, D.; Lopez-Fernandez, L.A.; Muñoz-Fernández, M. Carbosilane Dendrimers to Transfect Human Astrocytes with Small Interfering RNA Targeting Human Immunodeficiency Virus. BioDrugs 2010, 24, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Schipper, M.L.; Iyer, G.; Koh, A.L.; Cheng, Z.; Ebenstein, Y.; Aharoni, A.; Keren, S.; Bentolila, L.A.; Li, J.; Rao, J.; et al. Particle Size, Surface Coating, and PEGylation Influence the Biodistribution of Quantum Dots in Living Mice. Small 2009, 5, 126–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaeili, F.; Ghahremani, M.H.; Esmaeili, B.; Khoshayand, M.R.; Atyabi, F.; Dinarvand, R. PLGA nanoparticles of different surface properties: Preparation and evaluation of their body distribution. Int. J. Pharm. 2008, 349, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Zardini, A.A.; Mohebbi, M.; Farhoosh, R.; Bolurian, S. Production and characterization of nanostructured lipid carriers and solid lipid nanoparticles containing lycopene for food fortification. J. Food Sci. Technol. 2017, 55, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Talegaonkar, S.; Bhattacharyya, A. Potential of Lipid Nanoparticles (SLNs and NLCs) in Enhancing Oral Bioavailability of Drugs with Poor Intestinal Permeability. AAPS PharmSciTech 2019, 20, 121. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.; Thakkar, H. Darunavir-Loaded Lipid Nanoparticles for Targeting to HIV Reservoirs. AAPS PharmSciTech 2017, 19, 648–660. [Google Scholar] [CrossRef]
- Bhalekar, M.R.; Upadhaya, P.G.; Madgulkar, A.R.; Kshirsagar, S.J.; Dube, A.; Bartakke, U.S. In-vivo bioavailability and lymphatic uptake evaluation of lipid nanoparticulates of darunavir. Drug Deliv. 2015, 23, 2581–2586. [Google Scholar] [CrossRef] [PubMed]
- Stavudine Entrapped Lipid Nanoparticles for Targeting Lymphatic HIV Reservoirs—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21612153/ (accessed on 30 August 2020).
- Kuo, Y.-C.; Chung, J.-F. Physicochemical properties of nevirapine-loaded solid lipid nanoparticles and nanostructured lipid carriers. Colloids Surf. B Biointerfaces 2011, 83, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Mudassir, J.; Akhtar, S.; Murugaiyah, V.; Darwis, Y. Freeze-Dried Lopinavir-Loaded Nanostructured Lipid Carriers for Enhanced Cellular Uptake and Bioavailability: Statistical Optimization, in Vitro and in Vivo Evaluations. Pharmaceutics 2019, 11, 97. [Google Scholar] [CrossRef] [Green Version]
- Endsley, A.N.; Ho, R.J. Enhanced Anti-HIV Efficacy of Indinavir After Inclusion in CD4-Targeted Lipid Nanoparticles. JAIDS J. Acquir. Immune Defic. Syndr. 2012, 61, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Makwana, V.; Jain, R.; Patel, K.; Nivsarkar, M.; Joshi, A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: Elucidation of mechanism of uptake using chylomicron flow blocking approach. Int. J. Pharm. 2015, 495, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Bazzill, J.D.; Son, S.; Nam, J.; Shin, S.W.; Ochyl, L.J.; Stuckey, J.A.; Meagher, J.L.; Chang, L.; Song, J.; et al. Lipid-based vaccine nanoparticles for induction of humoral immune responses against HIV-1 and SARS-CoV-2. J. Control Release 2020, 330, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.O.; Pardi, N.; Parks, R.; Santra, S.; Mu, Z.; Sutherland, L.; Scearce, R.; Barr, M.; Eaton, A.; Hernandez, G.; et al. Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates. npj Vaccines 2021, 6, 50. [Google Scholar] [CrossRef]
- Bae, M.; Kim, H. The Role of Vitamin C, Vitamin D, and Selenium in Immune System against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Cathcart, R.F. Vitamin C in the treatment of acquired immune deficiency syndrome (AIDS). Med. Hypotheses 1984, 14, 423–433. [Google Scholar] [CrossRef]
- Cavalu, S.; Antoniac, I.V.; Mohan, A.; Bodog, F.; Doicin, C.; Mates, I.; Ulmeanu, M.; Murzac, R.; Semenescu, A. Nanoparticles and Nanostructured Surface Fabrication for Innovative Cranial and Maxillofacial Surgery. Materials 2020, 13, 5391. [Google Scholar] [CrossRef] [PubMed]
- Berretta, M.; Quagliariello, V.; Maurea, N.; Di Francia, R.; Sharifi, S.; Facchini, G.; Rinaldi, L.; Piezzo, M.; Manuela, C.; Nunnari, G.; et al. Multiple Effects of Ascorbic Acid against Chronic Diseases: Updated Evidence from Preclinical and Clinical Studies. Antioxidants 2020, 9, 1182. [Google Scholar] [CrossRef] [PubMed]
- Mallipeddi, R.; Rohan, L.C. Progress in antiretroviral drug delivery using nanotechnology. Int. J. Nanomed. 2010, 5, 533–547. [Google Scholar]
- Date, A.A.; Destache, C.J. A review of nanotechnological approaches for the prophylaxis of HIV/AIDS. Biomaterials 2013, 34, 6202–6228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, U.; Jain, N.K. Non-polymeric nano-carriers in HIV/AIDS drug delivery and targeting. Adv. Drug Deliv. Rev. 2010, 62, 478–490. [Google Scholar] [CrossRef] [PubMed]








| Class of Drug | Drug |
|---|---|
| Nucleoside Reverse Transcriptase Inhibitors (NRTIs) | Abacavir, Didanosine, Lamivudine, Stavudine, Zalcitabine, Zidovudine |
| Nucleoside Reverse Transcriptase Inhibitor (NRTIs) | Delavirdine, Efavirenz, Nevirapine |
| Nucleotide Reverse Transcriptase Inhibitors (NtRTIs) | Tenofovir diisoproxil fumarate |
| Protease Inhibitors (PIs) | Amprenavir, Indinavir, Lopinavir, Ritonavir, Nelfinavir, Saquinavir |
| Fusion Inhibitors (FIs) | Enfuvirtide |
| Co-Receptor Inhibitors (CRIs) | Maraviroc |
| Receptor | Ligands |
|---|---|
| d-Mannose | d-Mannose, fucose, N-acetyl glucose-mine, glucose, collagen, mannan, mannosyl lipoarabinomannan [103,104] |
| Folate | Folic acid [105,106] |
| Tuftsin | Tuftsin [107] |
| Scavenger | Modified LDL, lipopolysaccharides, lipoteichoic acid [108] |
| Transferrin | Transferrin [109] |
| Fc | Monoclonal Antibody [110] |
| Fibronectin | Fibronectin, laminin, serum amyloid P [117] |
| Toll-like receptor | LPS, lipoproteins, lipopeptides, and lipoarabinomannan [118] |
| Complement Receptors (CR3 and CR4) | C3b, iC3b, C3 [119] |
| Nanocarrier and Targeting Ligand | Drug | Targeting Sites |
|---|---|---|
| Liposomes | ||
| β-d-1-thiomannopyr-anoside | Indinavir | Liver, spleen, and lungs [120] |
| d-mannose | Stavudine | Maintained significant levels in the liver, spleen, and lungs and overcame the development of anemia and leukocytopenia [121] |
| Galactose | Stavudine | Prolonged residence in liver and spleen [122] |
| Galactose | Azidothymidine palmitate | Liver [123] |
| Galactose | Azidothymidine | Prolonged residence in the body [122] |
| d-mannose | Zidovudine | Lymph nodes and liver [124] |
| Antibodies against human and murine HLA-DR and CD4 antigen | Indinavir | Lymph nodes, liver, spleen, and plasma [101] |
| Nanoparticles | ||
| Transferrin | Azidothymidine | Brain [125,126] |
| Mannan | Didanosine | Spleen, lymph nodes, and brain [127] |
| d-mannose | Didanosine | Lung, liver, and lymph nodes [128] |
| Trans-Activating Transcriptor (TAT) peptide | Ritonavir | Brain [129] |
| SLN | ||
| Transferrin | Saquinavir | Brain microvascular endothelial cells [130] |
| Bovine serum albumin | Stavudine | Liver, spleen, brain [131,132] |
| Dextran | Stavudine | Liver, spleen, brain [132] |
| Drug | Particle Size | Targeting Sites |
|---|---|---|
| Liposomes | ||
| Stavudine | 120 ± 1.52 nm | Liver, spleen, and lungs [121] |
| Deoxycytidine | 300 nm | Reduced proviral DNA in mononuclear phagocyte system cells of spleen and bone marrow [173] |
| Foscarnet | Enhanced the drug localization in RES organs [144] | |
| 2′,3′-dideoxyinosine | 112 nm and 83 nm | Lymph nodes, liver, spleen [145] |
| Zidovudine | 130–160 nm | Lymph nodes, liver, spleen, plasma [174] |
| Zidovudine | 90 nm | Organs of RES and brain [175] |
| Zidovudine | 120 ± 10 nm | Spleen and lymph nodes [124] |
| Solid Lipid nanoparticles | ||
| Lopinavir-Ritonavir | 223 nm | Liver, spleen, mesenteric lymph nodes, and plasma [176,177] |
| Zidovudine | 181 ± 26 nm | Liver [123] |
| Lopinavir | 230 nm | Plasma and cerebrospinal fluid [16] |
| Zidovudine | 600–630 nm | Brain and liver [178] |
| Stavudine | 75 nm | Liver, spleen and lung [132] |
| Stavudine | 175 ± 6 nm | Liver, spleen, lungs, bone marrow, lymph nodes, and brain [179] |
| Efavirenz | 124.5–362 nm | Plasma [180] |
| Atazanavir | 167 nm | Enhanced accumulation in human brain microvessel endothelial cell line [181] |
| Polymeric nanoparticles | ||
| Zidovudine | 230 ± 20 nm | RES organs and plasma [182] |
| Indinavir | 1.6 um | Lung, liver, spleen, and bone marrow-derived macrophages [183] |
| Zidovudine | 56 to 93 nm | Brain, liver, and spleen [184] |
| Atazanavir | 268 nm | Liver and spleen [185] |
| Ritonavir, lopinavir and efavirenz | 331.2 ± 77.2 nm | Serum, brain, liver spleen, testes [176] |
| Efavirenz, lopinavir and ritonavir | 138.3 ± 55.4 nm | Enhanced cellular uptake and anti-HIV activity in H9 T cells [138] |
| Nevirapine | 450–550 nm | Brain, liver, and spleen [186] |
| Indinavir | 210 nm | Brain [187] |
| Rilpivirine | 200 nm | Sustained release in plasma [188] |
| Dendrimer | ||
| Lamivudine | ≈ 200 nm | Significantly enhanced uptake and anti-HIV activity [189] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotooh Abadi, L.; Damiri, F.; Zehravi, M.; Joshi, R.; Pai, R.; Berrada, M.; Massoud, E.E.S.; Rahman, M.H.; Rojekar, S.; Cavalu, S. Novel Nanotechnology-Based Approaches for Targeting HIV Reservoirs. Polymers 2022, 14, 3090. https://doi.org/10.3390/polym14153090
Fotooh Abadi L, Damiri F, Zehravi M, Joshi R, Pai R, Berrada M, Massoud EES, Rahman MH, Rojekar S, Cavalu S. Novel Nanotechnology-Based Approaches for Targeting HIV Reservoirs. Polymers. 2022; 14(15):3090. https://doi.org/10.3390/polym14153090
Chicago/Turabian StyleFotooh Abadi, Leila, Fouad Damiri, Mehrukh Zehravi, Rohit Joshi, Rohan Pai, Mohammed Berrada, Ehab El Sayed Massoud, Md. Habibur Rahman, Satish Rojekar, and Simona Cavalu. 2022. "Novel Nanotechnology-Based Approaches for Targeting HIV Reservoirs" Polymers 14, no. 15: 3090. https://doi.org/10.3390/polym14153090
APA StyleFotooh Abadi, L., Damiri, F., Zehravi, M., Joshi, R., Pai, R., Berrada, M., Massoud, E. E. S., Rahman, M. H., Rojekar, S., & Cavalu, S. (2022). Novel Nanotechnology-Based Approaches for Targeting HIV Reservoirs. Polymers, 14(15), 3090. https://doi.org/10.3390/polym14153090