1. Introduction
Current energy cell design needs to take many factors such as safety and energy density into consideration. The electrolyte acts as one of the most important parts in the energy storage device. As a medium, the electrolyte is placed between anode and cathode to help transfer ions in every working cycle of the battery. This novel polymer electrolyte required both excellent ion conduction and current separation in the same medium structure design, which normally was a thin membrane with hundreds of micrometer thickness [
1]. As a polymer electrolyte, this novel ceramic state electrolyte also performs a significant advantage over liquid electrolytes in terms of safety, weight, flexibility, and process ability.
Normally, the characterization of electronic and ionic conductivity is one of the most required steps in polymer electrolyte application [
2]. In the traditional process, this measurement can not avoid contacting the electrolyte sample with testing electrodes by using AC impedance spectroscopy [
3,
4,
5]. The measured complex impedance can be traced in the Nyquist plot shown in
Figure 1. The semicircle in the complex impedance plane could represent the ideal impedance of the electrolyte. With the characteristics of the Nyquist plot, the ionic conductivity can be derived by using an equivalent circuit model shown in
Figure 2.
The traditional contact measurement methods can not avoid contact impedance and error from partial contact problems because of physical contact between electrolyte and electrodes [
6,
7]. A series of newly developed non-contact dielectric measurements were firstly improved and applied in this novel ceramic state polymer electrolyte. The conductivity was successfully derived over a frequency range from 50 Hz to 20 KHz and from 8.2 GHz to 40 GHz.
The measurement in the frequency range 50 Hz to 20 KHz was using a capacitance bridge Andeen-Hagerling 2700A Bridge with an LD-3 dielectric capacitance cell. The measurements in the frequency range 8.2 GHz to 40 GHz were based on an Agilent 8510C vector network analyzer with eight different newly designed waveguides. The complex impedance spectra and complex conductivity spectra were derived from complex permittivity data obtained by these two measurements. With the same test process, the ionic relaxations can also be obtained by analyzing the loss tangent spectra.
2. Materials and Methods
A 12 mil (300 um) thickness electrolyte sample was prepared before the test. The whole series of experiments was processed in two frequency ranges, 50 Hz to 20 KHz and 8.2 GHz to 40 GHz. The measurement in these two frequency ranges used an air-gap capacitor method and in-waveguide method separately. Both methods are newly developed non-contact techniques. There was no physical contact on the electrolyte sample film surface during both measurements. The simplified diagram of both the measurement is shown below in
Figure 3.
Most electronic properties of electrolytes could be evaluated from their dielectric permittivity spectra. Normally the dielectric permittivity can be written as . Where ε′ is the real part of permittivity, which is also called the dielectric constant, ε′ is the imaginary part and is called dielectric loss.
The current density in electrolyte with an external electric field can be presented as:
where
Jconduction is the conduction current,
Jdisplacement is the displacement current, and is the complex conductivity. After transformation, we can calculate the complex conductivity as:
In this equation, σdc presents the electronic conductivity of the electrolyte at a direct current. For a solid polymer material, this part of conductivity is extremely small. The activation energy applied in the test would determine the coefficient n. Therefore, the dominant part in the complex conductivity formula is the imaginary part of permittivity. It is also the real part of complex AC conductivity σ’ in the same equation. This part is frequency dependent and has a DC ionic conductivity plateau in a frequency range. This value can be evaluated at the loss tangent peak to find the ionic conductivity of the polymer electrolyte.
Instead of directly measuring conductivity or impedance as in the traditional technique, capacitance, in this measurement, distance, transmittance, and reflectance were the direct measurement results. After that, the permittivity, loss tangent, and conductivity can be derived with these measured results.
In the two certain electromagnetic field environments in this paper, in the air gap inside the capacitor and space inside the electromagnetic waveguide, the electrolyte sample film was inserted and the input/output microwave signal was detected and analyzed to calculate the dielectric permittivity of the sample. The real part and imaginary part of complex permittivity can be used to deduce the conductivity of the sample then.
A newly designed sample holder and microwave waveguide were employed with an air-gap capacitor and vector network analyzer. The whole measurement was based on the electromagnetic field and microwave signal analysis. There was no physical contact between signal source electrodes and sample film surface during the test. The result measured by these non-contact methods is much more accurate because the contact impedance and weak contact error could be totally avoided. Furthermore, there are no chemical reactions between materials of electrolytes and electrodes.
- A
Air Gap Capacitor
For the measurement of the real part of permittivity (
ε′) and the imaginary part of permittivity (
ε″), an experimental setup is shown in
Figure 4. As an energy storage component, the
ε′ of electrolyte stands for its storage capability and
ε″ represent the loss of energy. Moreover, the loss tangent or dissipation factor was used to determine the ratio, tan
δ =
ε″/
ε′.
Compared to other chemical or physical methodology, this dielectric characterization can be much more easily processed. The convenience and accuracy make them a great measurement method for this kind of solid material. As we know, the real part of the dielectric constant can be calculated by ε′ = Cx/C0, where C0 is capacitance in a vacuum and Cx is capacitance with the inserted electrolyte. With this definition, we can easily conclude that electrolytes require high dielectric permittivity for better energy storage.
In practical measurements, the electrodes are normally pressed by clamping on the electrolyte sample to avoid a weak contact problem, however, the contact problem can not be totally avoided. In our non-contact measurement process, we prepared the specimen with a designed thickness for further accurate characterization. The average thickness of sample
hm would be measured by placing the specimen in some different positions, as shown in
Figure 5. During the test, the space between electrodes would be set to 100 to 200 um longer than the thickness of the sample, as
ha. Two groups of the capacitor and dissipation factor were measured with the sample inserted and without,
Cxa/tanδ
xa and
Ca/tanδ
a. The dielectric constant can be derived by:
The dissipation factor tanδ is calculated from
In this measurement, normal error in the contact method can be totally avoided. The accuracy in the preparation of specimen thickness hm, and measurement of holder space length ha becomes the main accuracy limitation factor. Repeating the measurement process with the sample placed in different position could significantly improve the accuracy.
For the measurement in frequency range from 8.2 GHz to 40 GHz, the in-waveguide measurement was setup as shown in
Figure 6. The diagram of how specimen placed was shown in
Figure 7. S-parameter was measured for the inserted sample [
8,
9].
Before the practical measurement, the reference planes need to be set by using TRL (thru-reflect-line) calibration program with the typical quarter wavelength difference (l). The S-parameters in this measurement can be calculated as:
In this equation, d is the thickness of the specimen to be tested, kc is the cutoff wavenumber, k0 is the sample’s wavenumber, and l refers to the quarter wavelength difference between thru and line. The effect of d is much smaller than the holder’s thickness was taken into consideration in this calculation.
This measurement setup can negate any undesired effect from the waveguide itself, because these calibration process can help achieve less than –50 dB return losses inside the waveguide. With the direct measurement results, the reflection and transmission can be derived as:
The transmission coefficient through the material may also be written as
T = e
−γd = e
−(α + jβ)d. The propagation constant through the material inside the waveguides has been derived to be:
After analyzing the above equations and boundary conditions, the propagation constant can present as:
The complex dielectric permittivity can be derived by utilizing these equations [
10]. The final permittivity in this measurement is shown as:
3. Results
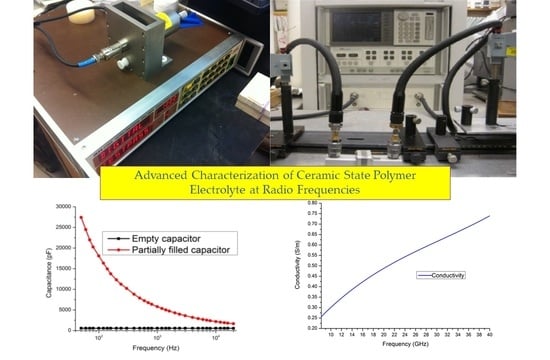
In measurements using the air-gap capacitance bridge technique, the capacitance and loss tangent spectra were derived and plotted in
Figure 8a,b. The data was collected and calculated in frequency from 50 Hz to 20 kHz. In measurement using the new designed in-waveguide technique, the reflection and transmittance can be measured. After calculation using collected data, the complex permittivity and conductivity can be derived and is shown in
Figure 9a,b and
Figure 10, separately. The final plot is close to a linear function with frequency increasing.
In this ceramic state polymer electrolyte, the ions would normally move in two ways, as local vibrations and diffusion [
11]. Because the external electric field changes rapidly in the high-frequency range like GHz, the ions in this field condition would not be motivated enough to transport. This performance makes the ions in the electrolyte become doing vibration more than diffusion [
12]. The relationship between ionic vibration and diffusion is shown as the slope of the plot in
Figure 10. The result of this series of measurements indicates this novel ceramic polymer electrolyte achieved high conductivity close to other liquid and gel electrolytes. The performance of this polymer electrolyte was better at low temperature because many kinds of liquid electrolytes have serious working temperature limitations. The Cole–Cole plot of this polymer electrolyte is shown in
Figure 11.
At low frequency, from dc to 100 Hz, the polymer electrolyte is required to perform high electrode polarization as an ionic conductive material [
13]. From the loss tangent plot in
Figure 8b, the peak was present at a frequency of around 2 KHz. For comparison with traditional ionic conductivity, the Nyquist plot is used to show the peak frequency is consistent. In polymer electrolytes, lithium ionic relaxation employs the ions as a single-ion carrier [
14]. Moreover, the loss tangent peak could help to analyze the ionic relaxation time and diffusion activation energy at a different temperature [
15]. Because the ionic relaxation is dominated in this solid electrolyte at a frequency from 8.2 GHz to 40 GHz, the dielectric permittivity decreases as the frequency goes up in this range.
4. Discussion
For verification of this newly developed non-contact characterization technique, the same sample was measured by the traditional contact method, as in
Figure 12, to make a comparison.
In the contact method, the sample film was held by a clamp with an AC source. The pressure on the clamp would obliviously affect the test result of the electrolyte because of weak contact errors. The conductivity measured by this method was about 30% lower than the non-contact method result. This is reasonable because the incomplete contact would result in more AC impedance. This weak contact impedance can be reduced by using better equipment with more stable pressure, however, this system error due to technique can not be fully avoided. The samples with different thickness, area, and roughness would result in a big difference in contacting force. The test result with this contact method has poor consistency and low reproducibility.
In contrast, the non-contact measurement could avoid the contact impedance and weak contact problem in traditional methods. However, there is still some uncertainty in both the air-gap capacitor and in-waveguide techniques. The random error of capacitance was 10−3 pF, and the random error of the dissipation factor was 10−5; both are much higher than what we expected from the instruments, which should be 10−5 pF and 1.5 × 10−7 for Andeen-Hagerling 2700A Bridge and loss tangent separately. In addition, the return loss in Agilent 8510C VNA was less than −50 dB which was worse than designed. This makes a 1% uncertainty belong to measured dielectric permittivity. These uncertainties mostly come from instrument accuracy limitations, which can be improved by upgrading measurement equipment in the future.