The Length Change Ratio of Ground Granulated Blast Furnace Slag-Based Geopolymer Blended with Magnesium Oxide Cured in Various Environments
Abstract
:1. Introduction
2. Material
2.1. GGBFS
2.2. Alkaline Activator
2.3. MgO
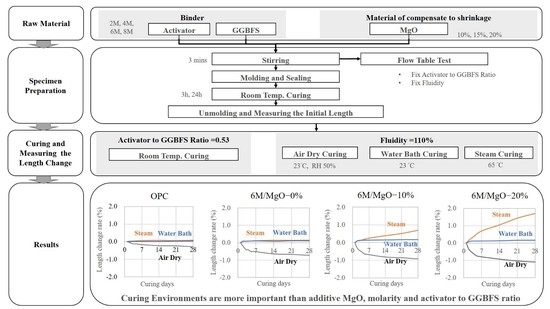
3. Experiment
3.1. Preparation of Specimen
3.2. Experimental Planning
3.3. Experimental Method and Equipment
4. Result and Discussion
4.1. Slurry Fluidity
4.2. Length Change Ratio
4.3. XRD Analysis
5. Machine Learning for Main Factors of Length Change Ratio
- If the specimen is air-dry cured, unmolded after three hours, and the MgO amount is more than or equal to 5%, then the expected length change ratio is −3.3%.
- If the specimen is air-dry cured, unmolded after three hours, and the MgO amount is less than 5%, then the expected length change ratio is −2.2%.
- If the specimen is room-temperature cured and demolded three hours later, then the expected length change ratio is −1.2%.
- If the specimen is air-dry or room-temperature cured and demolded 24 h later, then the expected length change ratio is −0.95%
- If the specimen is water-bath cured, then the expected length change ratio is 0.1%.
- If the specimen is steam cured and the MgO amount is less than 15%, then the expected length change ratio is 0.33%.
- If the specimen is steam cured and the MgO amount is more than or equal to 15%, then the expected length change ratio is 1.5%.
6. Conclusions
- When the activator to binder ratio was fixed, the shrinkage of the GP cured in an air-dry and room temperature environment was decreased with the addition of MgO.
- The workability of fresh GP slurry will reduce with the addition of MgO. It is necessary to increase the activator to binder ratio to maintain fluidity. However, the shrinkage of GP will increase.
- Comparing GP specimens unmolded at 3 h and 24 h, delaying the demolding time can reduce shrinkage.
- The XRD results indicate the shrinkage of GP cured in the air-dry environment cannot be compensated by MgO because there is not enough water to hydrate the MgO to form Mg(OH)2.
- The GP specimens cured in a moist environment (water-bath and steam) can avoid shrinkage and produce slight expansion, while the addition of MgO may cause over-expansion and fracture.
- Increasing the NaOH molarity of the activator from 2 M to 8 M can reduce the shrinkage of GP under air-dry curing, and can also increase the expansion under water bath can steam curing.
- The results of the statistical and machine learning analysis show that the length change ratio of GP is mainly affected by curing conditions and unmolding time, while the addition of MgO is not the main factor.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Price, L.; Lin, E. Emerging energy-efficiency and CO2 emission-reduction technologies for cement and concrete production: A technical review. Renew. Sustain. Energy Rev. 2012, 16, 6220–6238. [Google Scholar] [CrossRef]
- Wei, J.; Cen, K. Empirical assessing cement CO2 emissions based on china’s economic and social development during 2001–2030. Sci. Total Environ. 2019, 653, 200–211. [Google Scholar] [CrossRef]
- Najabat, A.; Abbas, J.; Muhammad, A.; Shazada, K.; Muhammad, A.; Adil, H.; Muhammad, R.; Xu, M. The greenhouse gas emissions produced by cement production and its impact on environment: A review of global cement processing. Int. J. Res. 2015, 2, 488–500. [Google Scholar]
- Alghamdi, H. A review of cementitious alternatives within the development of environmental sustainability associated with cement replacement. Environ. Sci. Pollut. Res. 2022, 29, 42433–42451. [Google Scholar] [CrossRef] [PubMed]
- Wojtacha-Rychter, K.; Kucharski, P.; Smolinski, A. Conventional and alternative sources of thermal energy in the production of cement—An impact on CO2 emission. Energies 2021, 14, 1539. [Google Scholar] [CrossRef]
- Vargas, J.; Halog, A. Effective carbon emission reductions from using upgraded fly ash in the cement industry. J. Clean. Prod. 2015, 103, 948–959. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary Portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Davidovits, J. Synthesis of new high-temperature geo-polymers for reinforced plastics/composites. In Proceedings of the PACTEC ’79 Society of Plastics Engineers, Costa Mesa, CA, USA, 31 January–2 February 1979; pp. 151–154. [Google Scholar]
- Davidovits, J. Why Alkali-Activated Materials (AAM) Are Not Geopolymers? Technical Paper #25; Geopolymer Institute Library: Saint-Quentin, France, 2018; Available online: www.geopolymer.org (accessed on 10 November 2018). [CrossRef]
- Davidovits, J. Solid-phase synthesis of a mineral blockpolymer by low temperature polycondensation of alumino-silicate polymers: Na-Poly(Sialate) or Na-PS and characteristics. In Proceedings of the Conference: IUPAC Symposium on Long-Term Properties of Polymers and Polymeric Materials. At: Stockholm, Sweden Volume: Topic III: New Polymers of High Stability, Stockholm, Sweden, 30 August–1 September 1976. [Google Scholar]
- Fernández-Jiménez, A.; Palomo, A.; Sobrados, I.; Sanz, J. The role played by the reactive alumina content in the alkaline activation of fly ashes. Microporous Mesoporous Mater. 2006, 91, 111–119. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, C.; Zhang, Z.; Wang, H.; Zhou, S.; Zhou, W. A comparative study on fly ash, geopolymer and faujasite block for pb removal from aqueous solution. Fuel 2016, 185, 181–189. [Google Scholar] [CrossRef]
- Marvila, M.T.; de Azevedo, A.R.G.; Vieira, C.M.F. Reaction mechanisms of alkali-activated materials. Rev. IBRACON De Estrut. Mater. 2021, 14, e14309. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer cements to minimize carbon dioxide greenhouse warming. Ceram. Trans. 1993, 37, 165–182. [Google Scholar]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Fahim Huseien, G.; Mirza, J.; Ismail, M.; Ghoshal, S.K.; Abdulameer Hussein, A. Geopolymer mortars as sustainable repair material: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 54–74. [Google Scholar] [CrossRef]
- Luhar, S.; Nicolaides, D.; Luhar, I. Fire resistance behaviour of geopolymer concrete: An overview. Buildings 2021, 11, 82. [Google Scholar] [CrossRef]
- Temuujin, J.; Rickard, W.; Lee, M.; van Riessen, A. Preparation and thermal properties of fire resistant metakaolin-based geopolymer-type coatings. J. Non-Cryst. Solids 2011, 357, 1399–1404. [Google Scholar] [CrossRef]
- Lahoti, M.; Tan, K.H.; Yang, E.-H. A Critical review of geopolymer properties for structural fire-resistance applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Youssef, N.; Rabenantoandro, A.Z.; Dakhli, Z.; Chapiseau, C.; Waendendries, F.; Hage Chehade, F.; Lafhaj, Z. Reuse of waste bricks: A new generation of geopolymer bricks. SN Appl. Sci. 2019, 1, 1252. [Google Scholar] [CrossRef]
- Madani, H.; Ramezanianpour, A.A.; Shahbazinia, M.; Ahmadi, E. Geopolymer bricks made from less active waste materials. Constr. Build. Mater. 2020, 247, 118441. [Google Scholar] [CrossRef]
- Lavanya, B.; Kuriya, P.D.; Suganesh, S.; Indrajith, R.; Chokkalingam, R.B. Properties of geopolymer bricks made with flyash and GGBS. IOP Conf. Ser. Mater. Sci. Eng. 2020, 872, 012141. [Google Scholar] [CrossRef]
- Kumar, R.; Das, P.; Beulah, M.; Arjun, H.R. Geopolymer bricks using iron ore tailings, slag sand, ground granular blast furnace slag and fly ash. In Geopolymers and Other Geosynthetic; Chapter 4; IntechOpen: London, UK, 2020. [Google Scholar]
- Tennakoon, C.; Shayan, A.; Sanjayan, J.G.; Xu, A. Chloride ingress and steel corrosion in geopolymer concrete based on long term tests. Mater. Des. 2017, 116, 287–299. [Google Scholar] [CrossRef]
- Gunasekara, C.; Law, D.; Bhuiyan, S.; Setunge, S.; Ward, L. Chloride induced corrosion in different fly ash based geopolymer concretes. Constr. Build. Mater. 2019, 200, 502–513. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.-B. Sulfate attack on alkali-activated slag concrete. Cem. Concr. Res. 2002, 32, 211–216. [Google Scholar] [CrossRef]
- Palomo, A.; Blanco-Varela, M.T.; Granizo, M.L.; Puertas, F.; Vazquez, T.; Grutzeck, M.W. Chemical stability of cementitious materials based on metakaolin. Cem. Concr. Res. 1999, 29, 997–1004. [Google Scholar] [CrossRef]
- Cheng, T.W.; Chiu, J.P. Fire-resistant geopolymer produced by granulated blast furnace slag. Miner. Eng. 2003, 16, 205–210. [Google Scholar] [CrossRef]
- Hadi, M.N.S.; Farhan, N.A.; Sheikh, M.N. Design of geopolymer concrete with ggbfs at ambient curing condition using Taguchi method. Constr. Build. Mater. 2017, 140, 424–431. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Ghoshal, S.K.; Ariffin, M.A.M. Effect of metakaolin replaced granulated blast furnace slag on fresh and early strength properties of geopolymer mortar. Ain Shams Eng. J. 2018, 9, 1557–1566. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Cracking tendency of alkali-activated slag concrete subjected to restrained shrinkage. Cem. Concr. Res. 2000, 30, 791–798. [Google Scholar] [CrossRef]
- Cartwright, C.; Rajabipour, F.; Radlińska, A. Shrinkage characteristics of alkali-activated slag cements. J. Mater. Civ. Eng. 2015, 27. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, L.; Yang, Y.; Gao, Y.; Yang, C. Effect of early age-curing methods on drying shrinkage of alkali-activated slag concrete. Materials 2019, 12, 1633. [Google Scholar] [CrossRef] [PubMed]
- Wallah, S.E. Drying shrinkage of heat-cured fly ash-based geopolymer concrete. Mod. Appl. Sci. 2009, 3, 14–21. [Google Scholar] [CrossRef]
- Duran Atiş, C.; Bilim, C.; Çelik, Ö.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Microcracking and strength development of alkali activated slag concrete. Cem. Concr. Compos. 2001, 23, 345–352. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Shrinkage mechanisms of alkali-activated slag. Cem. Concr. Res. 2016, 88, 126–135. [Google Scholar] [CrossRef]
- Kuenzel, C.; Vandeperre, L.J.; Donatello, S.; Boccaccini, A.R.; Cheeseman, C. Ambient temperature drying shrinkage and cracking in metakaolin-based geopolymers. J. Am. Ceram. Soc. 2012, 95, 3270–3277. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Zhao, J. Mitigating the drying shrinkage and autogenous shrinkage of alkali-activated slag by NaAlO2. Materials 2020, 13, 3499. [Google Scholar] [CrossRef]
- Mermerdaş, K.; Algın, Z.; Ekmen, Ş. Experimental assessment and optimization of mix parameters of fly ash-based lightweight geopolymer mortar with respect to shrinkage and strength. J. Build. Eng. 2020, 31, 101351. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Wang, R.; Chen, F.; Jia, X.; Cong, P. Effects of reactive mgo on the reaction process of geopolymer. Materials 2019, 12, 526. [Google Scholar] [CrossRef] [PubMed]
- Hanjitsuwan, S.; Injorhor, B.; Phoo-ngernkham, T.; Damrongwiriyanupap, N.; Li, L.-Y.; Sukontasukkul, P.; Chindaprasirt, P. Drying shrinkage, strength and microstructure of alkali-activated high-calcium fly ash using fgd-gypsum and dolomite as expansive additive. Cem. Concr. Compos. 2020, 114, 103760. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F. Effect of shrinkage-reducing admixtures on the properties of alkali-activated slag mortars and pastes. Cem. Concr. Res. 2007, 37, 691–702. [Google Scholar] [CrossRef]
- Dao, D.; Ly, H.-B.; Trinh, S.; Le, T.-T.; Pham, B. Artificial intelligence approaches for prediction of compressive strength of geopolymer concrete. Materials 2019, 12, 983. [Google Scholar] [CrossRef]
- Zou, Y.; Zheng, C.; Alzahrani, A.M.; Ahmad, W.; Ahmad, A.; Mohamed, A.M.; Khallaf, R.; Elattar, S. Evaluation of artificial intelligence methods to estimate the compressive strength of geopolymers. Gels 2022, 8, 271. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, W.; Aslam, F.; Joyklad, P. Compressive strength prediction of fly ash-based geopolymer concrete via advanced machine learning techniques. Case Stud. Constr. Mater. 2022, 16, e00840. [Google Scholar] [CrossRef]
- Wang, Q.; Ahmad, W.; Ahmad, A.; Aslam, F.; Mohamed, A.; Vatin, N.I. Application of soft computing techniques to predict the strength of geopolymer composites. Polymers 2022, 14, 1074. [Google Scholar] [CrossRef]
- Cao, R.; Fang, Z.; Jin, M.; Shang, Y. Application of machine learning approaches to predict the strength property of geopolymer concrete. Materials 2022, 15, 2400. [Google Scholar] [CrossRef] [PubMed]
- Bal, L.; Buyle-Bodin, F. Artificial neural network for predicting drying shrinkage of concrete. Constr. Build. Mater. 2013, 38, 248–254. [Google Scholar] [CrossRef]
- Kioumarsi, M.; Azarhomayun, F.; Haji, M.; Shekarchi, M. Effect of shrinkage reducing admixture on drying shrinkage of concrete with different w/c ratios. Materials 2020, 13, 5721. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C. Prediction of concrete strength using artificial neural networks. Eng. Struct. 2003, 25, 849–857. [Google Scholar] [CrossRef]
- Adesanya, E.; Aladejare, A.; Adediran, A.; Lawal, A.; Illikainen, M. Predicting shrinkage of alkali-activated blast furnace-fly ash mortars using artificial neural network (ANN). Cem. Concr. Compos. 2021, 124, 104265. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Khan, T.M.Y.; Dawood Abdul Khadar, S. Molarity Activity Effect on mechanical and microstructure properties of geopolymer concrete: A review. Case Stud. Constr. Mater. 2022, 16, e01014. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Nisar, K.S.; Abdel-Aty, A.-H.; Yahia, I.S. Evaluation of the effect of granite waste powder by varying the molarity of activator on the mechanical properties of ground granulated blast-furnace slag-based geopolymer concrete. Polymers 2022, 14, 306. [Google Scholar] [CrossRef] [PubMed]
- Memon, F.A.; Nuruddin, M.F.; Khan, S.; Shafiq, N.A.; Ayub, T. Effect of sodium hydroxide concentration on fresh properties and compressive strength of self-compacting geopolymer concrete. J. Eng. Sci. Technol. 2013, 8, 44–56. [Google Scholar]
- Rahman, S.H.B.A.; Irawan, S.; Shafiq, N.; Rajeswary, R. Investigating the expansion characteristics of geopolymer cement samples in a water bath and compared with the expansion of ASTM class-g cement. Heliyon 2020, 6, e03478. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.D.; Mobasher, B. Drying shrinkage of expansive cements. J. Mater. Sci. 1988, 23, 1976–1980. [Google Scholar] [CrossRef]
- Kurihara, R.; Maruyama, I. Effects of heating and drying on the strength and stiffness of high-early-strength Portland cement pastes. Cem. Concr. Compos. 2020, 106, 103455. [Google Scholar] [CrossRef]












| Composition | CaO | SiO2 | Al2O3 | MgO | SO2 | TiO2 | Fe2O3 | MnO2 | K2O | P2O5 | Cr2O3 | SrO | ZrO2 | Total |
| Percentage (%) | 47.32 | 32.43 | 9.96 | 5.16 | 2.29 | 0.80 | 0.60 | 0.55 | 0.41 | 0.29 | 0.02 | 0.11 | 0.06 | 100.00 |
| Composition | MgO | SiO2 | SO2 | CaO | K2O | P2O5 | TiO2 | MnO2 | Total |
| Percentage (%) | 94.67 | 2.22 | 1.52 | 0.89 | 0.34 | 0.27 | 0.05 | 0.03 | 100.00 |
| Unmolding Time | Group | Curing Environment | NaOH Molarity (M) | MgO (%) | Number of Specimen |
|---|---|---|---|---|---|
| 3 h | Fix A/B | Room Temp. | 6 | 0, 10, 15 | 9 |
| Fix Fluidity | Air-Dry | 4, 6, 8 | 0, 10, 20 | 27 | |
| Steam | 4, 6, 8 | 0, 10, 20 | 27 | ||
| Water bath | 4, 6, 8 | 0, 10, 20 | 27 | ||
| 24 h | Fix A/B | Air-Dry | 6 | 0, 10, 20 | 9 |
| Fix Fluidity | Air-Dry | 2, 4, 6 | 0, 10, 20 | 27 | |
| Steam | 2, 4, 6 | 0, 10, 20 | 27 | ||
| Water bath | 2, 4, 6 | 0, 10, 20 | 27 |
| Fixed | Curing Environment | NaOH Molarity | MgO (%) | A/B | Fluidity (%) | Length Change Ratio 1 (%) | Average of Length Change Ratio (%) |
|---|---|---|---|---|---|---|---|
| A/B | Room Temperature | 6 M | 0 | 0.50 | >200 2 | −1.521, −1.380, −1.666 | −1.522 |
| 10 | 155 | −1.244, −1.236, −1.325 | −1.268 | ||||
| 15 | 98 | −1.143, −0.670, −0.873 | −0.895 | ||||
| Fluidity | Air-Dry | 4 M | 0 | 0.35 | 110 ± 5 | −2.698, −2.606, −2.623 | −2.642 |
| 10 | 0.42 | −3.713, −3.739 | −3.726 | ||||
| 20 | 0.50 | −4.084, −3.972, −4.006 | −4.021 | ||||
| 6 M | 0 | 0.36 | −2.014, −1.956, −1.864 | −1.945 | |||
| 10 | 0.45 | −2.758, −2.841, −3.111 | −2.903 | ||||
| 20 | 0.53 | −3.768, −3.834 | −3.801 | ||||
| 8 M | 0 | 0.37 | −1.900, −1.854, −1.841 | −1.865 | |||
| 10 | 0.47 | −2.599, −2.558, −2.525 | −2.561 | ||||
| 20 | 0.56 | −3.209, −2.993, −3.082 | −3.095 |
| Fixed | Curing Environment | NaOH Molarity | MgO (%) | A/B | Fluidity (%) | Length Change Ratio 1 (%) | Average of Length Change Ratio (%) |
|---|---|---|---|---|---|---|---|
| A/B | Air-Dry | 6 M | 0 | 0.53 | >200 2 | −1.222, −1.165, −1.217 | −1.201 |
| 10 | 168 | −1.146, −1.181, −1.142 | −1.156 | ||||
| 20 | 113 | −0.906, −0.895, −0.879 | +0.893 | ||||
| Fluidity | Air-Dry | 2 M | 0 | 0.34 | 110 ± 5 | −1.015, −0.987, −0.976 | −0.993 |
| 10 | 0.41 | −1.140, −0.959, −1.162 | −1.087 | ||||
| 20 | 0.48 | −1.342, −1.430, −1.389 | −1.387 | ||||
| 4 M | 0 | 0.35 | −0.741, −0.744, −0.772 | −0.752 | |||
| 10 | 0.42 | −0.779, −0.790, −0.778 | −0.782 | ||||
| 20 | 0.50 | −0.946, −0.948, −0.925 | −0.940 | ||||
| 6 M | 0 | 0.36 | −0.723, −0.740, −0.721 | −0.728 | |||
| 10 | 0.45 | −0.944, −0.913, −0.930 | −0.929 | ||||
| 20 | 0.53 | −1.030, −1.022, −1.112 | −1.055 | ||||
| Steam | 2 M | 0 | 0.34 | +0.028, +0.036, +0.011 | +0.025 | ||
| 10 | 0.41 | +0.491, +0.498, +0.512 | +0.500 | ||||
| 20 | 0.48 | +1.557, +1.584, +1.571 | +1.571 | ||||
| 4 M | 0 | 0.35 | +0.098, +0.101, +0.090 | +0.096 | |||
| 10 | 0.42 | +0.501, +0.531, +0.510 | +0.514 | ||||
| 20 | 0.50 | +1.192, +1.269, +1.238 | +1.233 | ||||
| 6 M | 0 | 0.36 | +0.254, +0.132, +0.141 | +0.176 | |||
| 10 | 0.45 | +0.688, +0.744, +0.564 | +0.665 | ||||
| 20 | 0.53 | +1.698, +1.781, +1.814 | +1.764 | ||||
| Water bath | 2 M | 0 | 0.34 | +0.023, +0.013, +0.013 | +0.016 | ||
| 10 | 0.41 | +0.058, +0.053, +0.061 | +0.057 | ||||
| 20 | 0.48 | +0.088, +0.075, +0.089 | +0.084 | ||||
| 4 M | 0 | 0.35 | +0.076, +0.076, +0.086 | +0.079 | |||
| 10 | 0.42 | +0.113, +0.125, +0.131 | +0.123 | ||||
| 20 | 0.50 | +0.135, +0.150, +0.168 | +0.151 | ||||
| 6 M | 0 | 0.36 | +0.104, +0.102, +0.111 | +0.106 | |||
| 10 | 0.45 | +0.161, +0.139, +0.144 | +0.148 | ||||
| 20 | 0.53 | +0.154, +0.171, +0.167 | +0.164 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Lee, W.-H.; Cheng, T.-W.; Chen, W.; Li, Y.-F. The Length Change Ratio of Ground Granulated Blast Furnace Slag-Based Geopolymer Blended with Magnesium Oxide Cured in Various Environments. Polymers 2022, 14, 3386. https://doi.org/10.3390/polym14163386
Chen Y-C, Lee W-H, Cheng T-W, Chen W, Li Y-F. The Length Change Ratio of Ground Granulated Blast Furnace Slag-Based Geopolymer Blended with Magnesium Oxide Cured in Various Environments. Polymers. 2022; 14(16):3386. https://doi.org/10.3390/polym14163386
Chicago/Turabian StyleChen, Yen-Chun, Wei-Hao Lee, Ta-Wui Cheng, Walter Chen, and Yeou-Fong Li. 2022. "The Length Change Ratio of Ground Granulated Blast Furnace Slag-Based Geopolymer Blended with Magnesium Oxide Cured in Various Environments" Polymers 14, no. 16: 3386. https://doi.org/10.3390/polym14163386
APA StyleChen, Y. -C., Lee, W. -H., Cheng, T. -W., Chen, W., & Li, Y. -F. (2022). The Length Change Ratio of Ground Granulated Blast Furnace Slag-Based Geopolymer Blended with Magnesium Oxide Cured in Various Environments. Polymers, 14(16), 3386. https://doi.org/10.3390/polym14163386