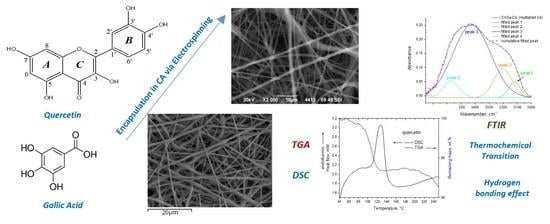
On the Thermochemical Transition Depression of Cellulose Acetate Composite Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Solutions and Electrospinning
2.3. Characterization
3. Results and Discussion
3.1. Thermal Behavior of Pure Substances
3.2. Composite Membranes
3.2.1. Cellulose Acetate-Quercetin Membranes
3.2.2. Cellulose Acetate-Gallic Acid Membranes
4. Further Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in cellulose ester performance and application. Prog. Polym. Sci. 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Cho, Y.; Kang, S.W. Porous cellulose acetate membranes prepared by water pressure-assisted process for water-treatment. J. Ind. Eng. Chem. 2019, 78, 421–424. [Google Scholar] [CrossRef]
- Markosyan, D.E.; Pirverdyan, A.I.; Mokhnachev, I.G.; Perepechkin, L.P. Cellulose acetate fibre for cigarette filters. Fibre Chem. 1971, 2, 292–293. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Lau, K.K.; Chew, T.L. Effect of spinning conditions on the fabrication of cellulose acetate hollow fiber membrane for CO2 separation from N2 and CH4. Polym. Test. 2019, 73, 1–11. [Google Scholar] [CrossRef]
- Lam, B.; Wei, M.; Zhu, L.; Luo, S.; Guo, R.; Morisato, A.; Alexandridis, P.; Lin, H. Cellulose triacetate doped with ionic liquids for membrane gas separation. Polymer 2016, 89, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Akbarzadeh, E.; Shockravi, A.; Vatanpour, V. High performance compatible thiazole-based polymeric blend cellulose acetate membrane as selective CO2 absorbent and molecular sieve. Carbohydr. Polym. 2021, 252, 117215. [Google Scholar] [CrossRef]
- Asiri, A.M.; Pugliese, V.; Petrosino, F.; Khan, S.B.; Alamry, K.A.; Alfifi, S.Y.; Marwani, H.M.; Alotaibi, M.M.; Mukherjee, D.; Chakraborty, S. Photocatalytic Degradation of Textile Dye on Blended Cellulose Acetate Membranes. Polymers 2022, 14, 636. [Google Scholar] [CrossRef] [PubMed]
- Cindradewi, A.W.; Bandi, R.; Park, C.-W.; Park, J.-S.; Lee, E.-A.; Kim, J.-K.; Kwon, G.-J.; Han, S.-Y.; Lee, S.-H. Preparation and Characterization of Cellulose Acetate Film Reinforced with Cellulose Nanofibril. Polymers 2021, 13, 2990. [Google Scholar] [CrossRef]
- Mousa, H.M.; Hussein, K.H.; Sayed, M.M.; Abd El-Rahman, M.K.; Woo, H.-M. Development and Characterization of Cellulose/Iron Acetate Nanofibers for Bone Tissue Engineering Applications. Polymers 2021, 13, 1339. [Google Scholar] [CrossRef]
- Rajesha, B.J.; Vishaka, V.H.; Balakrishna, G.R.; Padaki, M.; Nazri, N.A.M. Effective composite membranes of cellulose acetate for removal of benzophenone-3. J. Water Process Eng. 2019, 30, 100419. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Park, W.H. Antimicrobial cellulose acetate nanofibers containing silver nanoparticles. Carbohydr. Polym. 2006, 65, 430–434. [Google Scholar] [CrossRef]
- Faria, J.; Dionísio, B.; Soares, Í.; Baptista, A.C.; Marques, A.; Gonçalves, L.; Bettencourt, A.; Baleizão, C.; Ferreira, I. Cellulose acetate fibres loaded with daptomycin for metal implant coatings. Carbohydr. Polym. 2022, 276, 118733. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose acetate electrospun nanofibers for drug delivery systems: Applications and recent advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Pendas, S.; Asiminas, A.; Katranidis, A.; Tsioptsias, C.; Pitou, M.; Papadopoulos, G.; Choli-Papadopoulou, T. SpAD Biofunctionalized Cellulose Acetate Scaffolds Inhibit Staphylococcus aureus Adherence in a Coordinating Function with the von Willebrand A1 Domain (vWF A1). J. Funct. Biomater. 2022, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Kontogiannopoulos, K.N.; Assimopoulou, A.N.; Tsivintzelis, I.; Panayiotou, C.; Papageorgiou, V.P. Electrospun fiber mats containing shikonin and derivatives with potential biomedical applications. Int. J. Pharm. 2011, 409, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.S.; Mamdouh, W.; Nasr, M.; ElShaer, A.; Polycarpou, E.; Abdel-Aziz, R.T.A.; Sammour, O.A. Quercetin loaded cosm-nutraceutical electrospun composite nanofibers for acne alleviation: Preparation, characterization and experimental clinical appraisal. Int. J. Pharm. 2022, 612, 121309. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, A.; Sumnu, G.; Sahin, S. Fabrication of gallic acid loaded Hydroxypropyl methylcellulose nanofibers by electrospinning technique as active packaging material. Carbohydr. Polym. 2019, 208, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.H.A.; Salgado, H.R.N. Gallic Acid: Review of the Methods of Determination and Quantification. Crit. Rev. Anal. Chem. 2016, 46, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.A.; Cordeiro, A.M.T.M.; Damasceno, S.S.; Aguiar, R.T.; Rosenhaim, R.; Carvalho Filho, J.R.; Santos, I.M.G.; Maia, A.S.; Souza, A.G. Commercial antioxidants and thermal stability evaluations. Fuel 2012, 97, 638–643. [Google Scholar] [CrossRef]
- Daneshfar, A.; Ghaziaskar, H.S.; Homayoun, N. Solubility of Gallic Acid in Methanol, Ethanol, Water, and Ethyl Acetate. J. Chem. Eng. Data 2008, 53, 776–778. [Google Scholar] [CrossRef]
- Razmara, R.S.; Daneshfar, A.; Sahraei, R. Solubility of Quercetin in Water + Methanol and Water + Ethanol from (292.8 to 333.8) K. J. Chem. Eng. Data 2010, 55, 3934–3936. [Google Scholar] [CrossRef]
- Tsioptsias, C. Glass chemical transition: An unknown thermal transition observed in cellulose acetate butyrate. Carbohydr. Polym. 2021, 259, 117754. [Google Scholar] [CrossRef] [PubMed]
- Tsioptsias, C.; Nikolaidou, E.G.; Ntampou, X.; Tsivintzelis, I.; Panayiotou, C. Thermo-chemical transition in cellulose esters and other polymers. Thermochim. Acta 2022, 707, 179106. [Google Scholar] [CrossRef]
- Galwey, A.K.; Laverty, G.M. The thermal decomposition of dehydrated d-lithium potassium tartrate monohydrate: Molecular modification by a homogeneous melt mechanism. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1993, 440, 77–93. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Vincent, M.F.; Bright, F.V.; Liotta, C.L.; Eckert, C.A. Specific Intermolecular Interaction of Carbon Dioxide with Polymers. J. Am. Chem. Soc. 1996, 118, 1729–1736. [Google Scholar] [CrossRef]
- Condo, P.D.; Sanchez, I.C.; Panayiotou, C.G.; Johnston, K.P. Glass transition behavior including retrograde vitrification of polymers with compressed fluid diluents. Macromolecules 1992, 25, 6119–6127. [Google Scholar] [CrossRef]
- Lee, W.G.; Kim, D.H.; Jeon, W.C.; Kwak, S.K.; Kang, S.J.; Kang, S.W. Facile control of nanoporosity in Cellulose Acetate using Nickel(II) nitrate additive and water pressure treatment for highly efficient battery gel separators. Sci. Rep. 2017, 7, 1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Costa, E.M.; Filho, J.M.B.; do Nascimento, T.G.; Macêdo, R.O. Thermal characterization of the quercetin and rutin flavonoids. Thermochim. Acta 2002, 392–393, 79–84. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Karabinaki, O.; Christofilos, D.; Tzimpilis, E.; Tsivintzelis, I.; Panayiotou, C. On polymer-polymer miscibility and cellulose ester blends: A case study. Thermochim. Acta 2022, 714, 179265. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Sakellariou, K.G.; Tsivintzelis, I.; Papadopoulou, L.; Panayiotou, C. Preparation and characterization of cellulose acetate–Fe2O3 composite nanofibrous materials. Carbohydr. Polym. 2010, 81, 925–930. [Google Scholar] [CrossRef]
- Shen, Y.; Gallet-Pandellé, A.; Kurita, H.; Narita, F. Fabrication, Tensile Properties, and Photodecomposition of Basalt Fiber-Reinforced Cellulose Acetate Matrix Composites. Polymers 2021, 13, 3944. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.M.; Petrosino, F.; Pugliese, V.; Khan, S.B.; Alamry, K.A.; Alfifi, S.Y.; Marwani, H.M.; Alotaibi, M.M.; Algieri, C.; Chakraborty, S. Synthesis and Characterization of Blended Cellulose Acetate Membranes. Polymers 2022, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Boughdiri, A.; Ounifi, I.; Chemingui, H.; Ursino, C.; Gordano, A.; Zouaghi, M.O.; Hafiane, A.; Figoli, A.; Ferjani, E. A preliminary study on cellulose acetate composite membranes: Effect of nanoparticles types in their preparation and application. Mater. Res. Express 2021, 9, 015003. [Google Scholar] [CrossRef]
- Bartenev, G.M. Weak chemical bonds and chemical relaxation and rupture processes in polymers. Polym. Sci. U.S.S.R. 1984, 26, 1855–1861. [Google Scholar] [CrossRef]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; John Wiley and Sons Ltd.: West Sussex, UK, 2004. [Google Scholar]
- Atkins, P.W.; De Paula, J.; Keeler, J. Atkins’ Physical Chemistry, 11th ed.; Oxford University Press: New York, NY, USA, 2018. [Google Scholar]
- Cremer, D.; Wu, A.; Larsson, A.; Kraka, E. Some Thoughts about Bond Energies, Bond Lengths, and Force Constants. Mol. Modeling Annu. 2000, 6, 396–412. [Google Scholar] [CrossRef]
- Zhao, L.; Zhi, M.; Frenking, G. The strength of a chemical bond. Int. J. Quantum Chem. 2022, 122, e26773. [Google Scholar] [CrossRef]






| Sample | Thermochemical Transition Temperature, °C | Mass Loss in the Range of 40–100 °C, wt.% | Mass Loss in the Range of 140–250 °C, wt.% |
|---|---|---|---|
| CA membrane | 220 | 2.2 | 1.0 |
| CA + 1.5% quercetin | 217 | 1.7 | 1.0 |
| CA + 3% quercetin | 216 | 1.3 | 1.1 |
| CA + 5% quercetin | 210 | 1.5 | 2.6 |
| Quercetin | >250 | 1.3 | 0.5 |
| Sample | Thermochemical Transition Temperature, °C | Mass Loss in the Range of 40–100 °C, wt.% | Mass Loss in the Range of 140–250 °C, wt.% |
|---|---|---|---|
| CA membrane | 220 | 2.2 | 1.0 |
| CA + 1% gallic acid | 216 | 0.7 | 1.0 |
| CA + 3% gallic acid | 210 | 0.9 | 1.5 |
| CA + 5% gallic acid | 205 | 0.9 | 3 |
| Gallic acid | 261 | 3 | 7.2 |
| Fitted Peak | Wavenumber, cm−1 | Area | % Area |
|---|---|---|---|
| 1 | 3119 | 5.90 | * |
| 2 | 3204 | 16.54 | 15 |
| 3 | 3424 | 86.34 | 78 |
| 4 | 3570 | 8.18 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsioptsias, C.; Foukas, G.-R.P.; Papaioannou, S.-M.; Tzimpilis, E.; Tsivintzelis, I. On the Thermochemical Transition Depression of Cellulose Acetate Composite Membranes. Polymers 2022, 14, 3434. https://doi.org/10.3390/polym14163434
Tsioptsias C, Foukas G-RP, Papaioannou S-M, Tzimpilis E, Tsivintzelis I. On the Thermochemical Transition Depression of Cellulose Acetate Composite Membranes. Polymers. 2022; 14(16):3434. https://doi.org/10.3390/polym14163434
Chicago/Turabian StyleTsioptsias, Costas, George-Romanos P. Foukas, Savvina-Maria Papaioannou, Evangelos Tzimpilis, and Ioannis Tsivintzelis. 2022. "On the Thermochemical Transition Depression of Cellulose Acetate Composite Membranes" Polymers 14, no. 16: 3434. https://doi.org/10.3390/polym14163434
APA StyleTsioptsias, C., Foukas, G. -R. P., Papaioannou, S. -M., Tzimpilis, E., & Tsivintzelis, I. (2022). On the Thermochemical Transition Depression of Cellulose Acetate Composite Membranes. Polymers, 14(16), 3434. https://doi.org/10.3390/polym14163434