1. Introduction
The rapid development of nanoscience and nanotechnology in recent decades has led to significant advances in the applications of nanomaterials in many technological fields. Thus, the special physical and chemical properties of nanomaterials have been widely explored to develop or improve applications aimed at solving important current global challenges, such as environmental remediation, sustainable energy, and climate change. Indeed, a great deal of scientific activity has been developed to obtain nanomaterials able to contribute, for example, to capture and efficiently use carbon dioxide from the air, degrading toxic pollutants in water and contributing to process of energy conversion and storage [
1,
2,
3]. Thus, the use of catalytic materials can help to improve the efficiency of diverse processes, their economy, and even contribute to reduce the generation of waste and pollutants. In this context, the exploration and development of advanced materials to be used in nanocatalysis represents an important challenge in the short-medium term, in order to contribute to sustainable chemistry [
4].
Thanks to the important achievements of nanoscience and nanotechnology, it is now possible to prepare with great precision many catalytic materials at the nanometer scale, achieving a significant improvement in their performance thanks to the so-called “nanoeffects”. These nanoeffects, although not fully understood to date, are attributed to structural, electronic and quantum size effects [
5]. Nanocatalysts can be formed by composites, compounds, alloys, or elemental solids and can also be obtained in different formats (e.g., powder, thin films, fibers). Among the nanomaterials with catalytic properties, nanoparticles (NPs) of noble metals stand out, since, in addition to providing high surface areas favoring the interaction between the reactants and the catalyst surface, they display their unique chemical, electronic, and optical properties through the well-known plasmonic effect [
6].
Notwithstanding the above, the use of nanomaterials as catalysts also presents some difficulties. For example, a considerable decrease in the activity of catalytic NPs has been observed after a few cycles of use, which has been attributed to aggregation and surface passivation phenomena. Indeed, when NPs agglomerate, they tend to lose their nanometer size and transform into bulk materials losing their special properties. Another problem really challenging is the difficulty to recover the catalytic nanoparticles from the reaction medium for further use. To solve these problems a suitable strategy is to support the nanoparticles on structures of different type and nature [
7], achieving that the supports not only facilitate the recovery and reuse of the nanocatalyst, but also prevent the aggregation of the NPs during successive reaction cycles or under continuous flow conditions. Interestingly, with the advancement of knowledge it has been established that in many cases the role played by the supports in practice may be rather more complex and even not yet clearly understood. Some supports can perform, directly or indirectly, different functions during a catalytic reaction, providing specific defect sites. These zones would allow to anchor and even stabilize metal nanoparticles favoring activity and transfer of electrons [
8].
In general, it is desirable that the ideal supports are chemically inert, have a large surface area and uniform size, and retain the nanocatalyst efficiently [
9]. Among the most used supports for nanocatalyst immobilization are inorganic fibers, glass mats, and different polymers [
10]. An alternative that has shown great promise for supporting and stabilizing nanoparticles, preserving their special properties over time and after repeated use in different chemical environments, is the use of polymeric nanofibers (NFs) [
11,
12]. One of the advantages offered by polymeric NFs is that these materials can be obtained on a macroscopic scale in the form of mat-like structures. These mats are characterized by high flexibility and elasticity, larges specific surface area, and show high degree of porosity, of small pore sizes and high pore interconnectivity. In addition, it has been demonstrated that electrospun nanofiber films can be modified and grafted with different functionalities, providing them with a high potential to be used for various purposes. Thanks to these special properties, polymeric nanofiber mats can be advantageously used in a variety of emerging environmental applications, such as air filtration, heavy metal ion adsorption, self-cleaning applications, and water purification processes. Consequently, considerable effort has been made to obtain hybrid catalytic nanomaterials of the Polymer Nanofibers containing metal Nanoparticles, in which the special characteristics of both nanocatalysts and nanofibers converge [
12,
13].
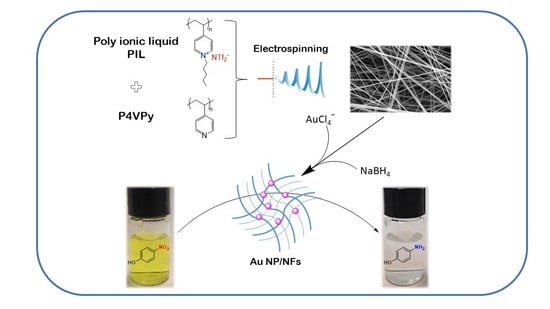
Inspired by the above, we have prepared a hybrid nanomaterial consisting of polymeric nanofibers and gold nanoparticles, and we studied its performance as a heterogeneous catalyst using the well-known reduction reaction of 4-nitrophenol (4NP) as a model. Noticeably, in this simple reduction reaction with sodium boron hydride catalyzed by gold nanoparticles, 4-NP, a compound considered a priority pollutant by the USEPA, is effectively reduced to form the considerably less toxic compound 4-aminephenol (4-AP) under mild reaction conditions, and the reaction can be easily monitored by UV-vis spectroscopy [
14,
15].
The polymeric nanofibers were obtained by electrospinning mixtures of the poly ionic liquid, Poly penthyl 4-vinylpyridine bis trifluoromethane sulfonamide (PP4VPy-NTf
2−) and poly 4-vinyl pyridine PV4Py, while the gold nanoparticles were synthesized in situ on the nanofibers by reduction in ionic precursors adsorbed on them. The polymeric ionic liquids PILs, also known as poly (ionic liquids) are a special type of polyelectrolytes that contain some of the chemical groups of ILs, cations and anions. PILs keep some of the special properties of Ils, such as, negligible vapor pressure, ionic conductivity, thermal stability, tuneable solution properties, nonflammability, and high chemical and electrochemical stabilities, as well as good compatibility. Many of the special properties that characterize ILs are mainly attributable to their ionic nature and are, therefore, also present in PILs. A particularly interesting property of PILs is their high capacity to interact and their compatibility with different materials, both organic and inorganic, hybrids, e.g., metal-organic and biomacromolecules [
16,
17]. Based on the above, PILs are expected to present a good performance as support for catalytic Au nanoparticles, mainly due to the potential generation of favorable interactions between NFs and NPs, providing high stability to the system along with other special properties such as flexibility, processability and mechanical durability, all highly desirable for supported catalysts. Therefore, the main novelty and also the main aim of this work lies in the inclusion of a PIL in the nanofibers and the study of its effect in the in situ gold nanoparticles and in the catalytic performance of the final hybrid nanomaterial. To the best of our knowledge, to date, information on the use of PIL-containing NFs as supports for catalytic NPs is at least scarce, so we seek to contribute to knowledge in this interesting area.
3. Results and Discussion
For the poly ionic liquid PP4VPy-NTf
2− synthesis, initially, P4VPy was reacted with an excess of pentyl bromide (alkylating agent) to achieve almost complete quaternization of the pyridinyl groups. A quaternization degree of 99% was obtained by FTIR and
1H-NMR analysis, as reported in a previous work [
18]. Then, the exchange of bromide for NTf
2− anions, was performed by direct dissolution of the LiNTf
2 salt on a solution of the polyelectrolyte PP4VPy-Br [
20]. Finally, purification of PP4VPy-NTf
2− PIL was performed by consecutive dialysis, filtration and lyophilization. The successful synthesis of PP4VPy-NTf
2− was corroborated by FTIR,
1H-NMR and
19F-NMR product characterization. A scheme of synthesis route and the corresponding spectra are shown in
Figure 1.
The FTIR spectrum (
Figure 1B) shows the typical bands expected for the quaternized PP4VPy
+ backbone, accompanied by new ones ascribed to vibrational modes of NTf
2− counterions. The quaternization process was verified by the appearance of an intense band at 1647 cm
−1 corresponding to the vibrational modes of the quaternized pyridine rings, and the absence of the signal attributed to the vibrations of the uncharged pyridine rings which typically appears at 1600 cm
−1. Moreover, the increased intensity of bands between 2800–3000 cm
−1, would denote a higher aliphatic content in the polymer due to the incorporation of pentyl moieties. On the other hand, the absorption bands at 1350, 1250, 1205, 1130, and 1050 cm
−1, are ascribed to NTf
2− counterions.
Figure 1C shows the
1H-NMR spectrum of PP4VPyNTf
2−, the most relevant signals are, at 8.74 and 7.38 ppm corresponding to protons of the quaternized pyridine rings, and signals at 1.81, 1.34, and 0.91 ppm protons of pentyl chains attached to the nitrogen atom of pyridine groups. Notably, the analysis of
1H-NMR spectrum indicated a quantitative polymer quaternization. Similarly, the
19F-NMR spectrum analysis allowed corroborate the presence of NTf
2− counterions (specifically, −CF
3 signal at 78.65 ppm) in the PIL structure.
Polyelectrolytes are among the most difficult polymers to be electrospun since this type of polymer has special properties in solution due to the presence of charges along their chains [
21]. To minimize this difficulty, nanofibers were obtained by electrospinning employing solutions prepared from mixtures of PP4VPy-NTf
2− and P4VPy in different proportions, using DMF/DCM (1:1
v/
v) as solvent.
Figure 2 shows SEM images of the obtained nanofibers obtained from the P4VPy and P4VPy/PP4VPyNTf
2− blends.
Images revealed the obtainment of uniform and bead-free nanofibers using pure P4VPy and their blends with compositions up to 30%
w/
w of PP4VPyNTf
2−. In contrast, the blend containing 50%
w/
w of PIL produced a material consisting of a high content of beads connected to each other by rather irregular fibers. A significant detriment of the mechanical properties of fibrous materials accompanied by a drastic effect on their surface properties due to the presence of beads have been reported [
22]. Notably, by increasing the amount of PIL in blends, mats with higher fiber density and lower fiber diameter values were obtained,
Figure 3A. In addition, the analysis of the size distributions shows that the diameter of the nanofibers becomes narrower as the PIL content in the electrospun solution increases. These results are ascribed to the increase in conductivity of the solutions as the PIL content increases (insert
Figure 3A), giving rise to an increase in the repulsive forces in the solution jet during the electrospinning process, phenomenon that significantly reduce the diameter of the fibers [
23]. On the other hand, a proper relation between the PIL content of solutions and obtained nanofibers was determined by means of EDX analysis, with a rising trend for the values of relative content of fluorine regarding carbon (% F/% C), as is shown in
Figure 3B.
The nanofibers obtained with different amounts of PIL were characterized by FTIR spectroscopy and thermogravimetric analysis (TGA).
Figure 4A shows the FTIR spectra of bare P4VPy NFs and of all P4VPy/PP4VPyNTf
2− blend NFs. The characteristic signal centered at 1600 cm
−1 (signal 2 in spectra) which correspond to vibrations of the non-quaternized pyridine rings, are observed in all nanofibers. Additionally, new signals ascribed to PIL are detected. As in the PIL spectrum (
Figure 1B), the FTIR spectra of the P4VPy/PP4VPyNTf
2− NFs present a signal attributed to the quaternized pyridine rings, labelled as 1, and signals corresponding to the NTf
2− counterions, from 3 to 7. As expected, these signals showed an intensity increase in those blends having higher PIL content.
Figure 4B,C show the thermal degradation profiles and their corresponding differential curves of the nanofibers of P4VPy and P4VPy/PP4VPyNTf
2− blends. From these results, relevant information such as onset degradation temperatures (T
onset), maximum decomposition rate temperatures (T
MAX), and percentage of residues were obtained. The above-mentioned values are summarized in
Table 1.
All samples showed an initial weight loss at around 100 °C, which is attributed to the removal of water molecules. All nanofibers except P4VPy exhibited a multistage degradation profile consisting of three stages. Reported works indicate that the first stage could be related to the degradation of the quaternized pyridinyl moieties since, apparently, the quaternization process induces a selective labilization of the pyridine structure [
24]. On the other hand, the second stage would correspond to the decomposition of pentyl chains. Interestingly, the temperatures of this stage in the blends containing 20 and 30% PIL are lower than that of blend containing 10% of PIL. The loss weight of third stage, would be related to the volatilization of fragments coming from NTf
2− counterions [
25]. This is consistent with the intensity increase in DTGA curves as the PIL content increases. Although the incorporation of PIL tends to reduce the onset temperature of NFs decomposition, all nanofiber mats showed good thermal stability by degrading above 300 °C, allowing them to be considered as suitable materials for a wide spectrum of applications.
Once the characterization of the nanofibers was completed, their capacity to adsorb Au (III), in the form of the AuCl
4− anions, was studied, in order to subsequently obtain gold nanoparticles “in situ” as has been reported in previous works [
26]. The kinetic of AuCl
4− adsorption onto P4VPy NFs, and NFs with different PIL contents was analyzed by plotting the adsorbed capacity (q) onto the P4VPy/PP4VPyNTf
2− nanofibers over time,
Figure 5.
From the above Figure, it can be seen that all nanofibers containing PIL showed a faster adsorption rate than P4VP NFs in the early stage of the process. In this sense, while P4VPy NFs required around 70 min to reach a plateau value, all PIL-based nanofibers achieved the above situation in remarkably less time. Additionally, an increase in the adsorption capacity was observed as the PIL content in nanofibers rises. Therefore, the inclusion of PIL proved to be an effective strategy to improve the AuCl4- adsorption process both kinetically and in terms of capacity.
To better understand the adsorption mechanism governing the coordination of gold ions, the experimental data shown in
Figure 6 were fitted using the well-known linear expression of a pseudo-first order kinetic model (Equation (2)).
In this equation,
t corresponds to the contact time between nanofibers and the AuCl
4− solution,
qt and
qe are the adsorption capacity at time t and at the equilibrium state (mg/g), respectively, while
k1 (min
−1) is the pseudo first-order kinetic constant. The main kinetic parameters, such as
k1 and
qe, are summarized in
Table 2.
Note that kinetic constant and q
e values increase as the PIL content in the nanofibrous material increases. Notably, the sample containing 30% PIL, in addition to being able to adsorb a higher amount of gold ions, exhibited a kinetic constant around six times higher than the one measured for P4VPy nanofibers. A plausible explanation to the previous results could be related to an increase in the charged species along the surface of the nanofibers containing the PP4VPyNTf
2− PIL, generating a faster adsorption of the ionic species due to electrostatic interactions. It is even reasonable to speculate the existence of an anion exchange process in which the NTf
2− entities of the PIL are replaced by AuCl
4− species as new counterions. To verify this hypothesis, XPS measurements of PP4VPyNTf
2− NFs before and after AuCl
4− adsorption were performed, and the changes in the elemental composition of the nanofiber surface were analyzed.
Figure 6 shows the survey XPS spectra belonging to PP4VPyNTf
2− (30%).
The XPS spectra show the main signals corresponding to the atoms that are part of the P4VPy and PP4VPyNTf2−. As expected, the appearance of the Au 4f signal is observed after the adsorption of gold ions occurs, corresponding to the Au 4f7/2 and 4f5/2 doublet at 83.1 eV and 87.0 eV, respectively. In addition, it is interesting to note the intensity decrease ascribed to the F 1s and S 2p signals, which would evidence a substitution of the NTf2− counterion upon adsorption of complex gold species on the surface of the fibers, suggesting an ion exchange mechanism.
To achieve a deeper understanding of the adsorption mechanism, a more detailed analysis of the main atoms present in the nanofibers XPS spectrum was performed with a particular interest in the region corresponding to nitrogen 1s. This is based on reports that have previously suggested that the N atom of the pyridinic ring has significant interactions with gold atoms [
27].
Figure 7 shows that the nitrogen region exhibits multiple signals that can be deconvoluted into signals with binding energies of 400.1, 397.2, and 396.6 eV, being assigned to nitrogen atoms present in quaternized pyridine rings (N
+). In addition, it is interesting to note the intensity decrease ascribed to non-quaternized pyridine rings (N) and the counterion specie (N
−), respectively. After gold ion adsorption, the nitrogen region undergoes remarkable changes, although the deconvolution of this region again indicated the presence of three different nitrogen species, N
+, N, and N
−, in this case the signals appear 398.4, 397.0, 396.5 eV, respectively. Thus, the most noticeable changes in the region of the XPS spectrum of nitrogen are, the shift of the N
+ signal to lower binding energies, and a decrease in the intensity of the N
− signal. Consequently, these results would indicate the involvement of N
+ during the adsorption process, along with the loss of some anionic species during the process. Therefore, according to the previous analysis it is possible to hypothesize that the adsorption would take place by an ionic exchange of NTf
2− by AuCl
4− species.
Once the AuCl4− adsorption capacity of the NFs was determined, the nanofibers containing adsorbed AuCl4− were subjected to a reduction process for the in situ generation of gold nanoparticles. This experiment was carried out adsorbing 1% w/w of Au+3 considering the total mass of the nanofiber film, in order to ensure the same amount of metal in all prepared nanocomposites.
After demonstrating the coordination capacity of these materials, the obtained nanofibers containing adsorbed AuCl4− species were tested in the elaboration of nanocomposites by reducing the gold precursor into metal nanoparticles. The experiments consisted of the treatment of nanofiber mat samples containing 1% w/w gold ions with a sodium borohydride solution, as described in the experimental section.
To observe the nanoparticles obtained by SEM, the organic fraction of the nanocomposites obtained was removed by calcining the nanofibers containing the nanoparticles by controlled heating up to 900 °C and depositing the residue obtained on a copper grid. The obtained images of the nanoparticles and the analysis of the corresponding size distribution are shown in
Figure 8. The size of nanoparticles synthesized in situ on pure P4VPy nanofibers was approximately 43 nm with a broad size distribution, while interestingly, when the amount of PIL in NFs increases, the nanoparticle size decreases to a minimum average size of 36 nm in the case of fibers containing 30% PP4VPy-NTf
2−. Notably, along with the decrease in nanoparticle size, the nanoparticles also exhibited a narrower particle size distribution as the PIL content increased.
Finally, as detailed in experimental section, the performance of nanofibers with different PIL contents and containing gold nanoparticles as heterogeneous catalysts was evaluated. The recognized model catalytic reaction to test the catalytic activity of metal nanoparticles, reduction in 4-nitrophenol to 4-aminophenol by sodium borohydride, was used. This reduction reaction can be easily followed by UV-visible spectroscopy, due to the characteristic light absorption presented by 4-nitrophenolate species at 400 nm [
28].
The kinetic data were determined using a pseudo-first order kinetic model, as Equation (3) indicates.
where [4
NP]
0 and [4
NP]
t correspond to 4-NP concentration at the beginning of the reaction and at t minutes after this was started, respectively,
t is the reaction time, and
kapp is the pseudo first-order kinetic constant of the reaction. Using the Lambert–Beer relation, Equation (3) can be converted to Equation (4).
where
Abst and
Abs0 correspond to 4-
NP absorbance at the beginning of the reaction and at t minutes after this started, respectively.
The results obtained, conversion profiles, pseudo first order models, and
kapp values, both for pure P4VPy nanofibers and with different poly liquid ionic contents, containing in all cases gold NPs, are shown in
Figure 9.
In all cases, a rapid increase in the conversion percentage was initially observed, which slowed down as time progressed, until the highest conversion values were reached after 140 min of reaction. Interestingly, the highest value of 4 NP to 4 AP conversion (96%) was obtained with the nanofibers with the highest PIL content.
Additionally, although the values of the kinetic constants are of the same order, an evident increase in the magnitude of kapp, corresponding to 10.5%, was observed for the reaction catalyzed with the nanofibers with 30% w/w of PIL and gold NPs, with respect to those with only P4VPy and gold NPs.