Bone Healing in Rat Segmental Femur Defects with Graphene-PCL-Coated Borate-Based Bioactive Glass Scaffolds
Abstract
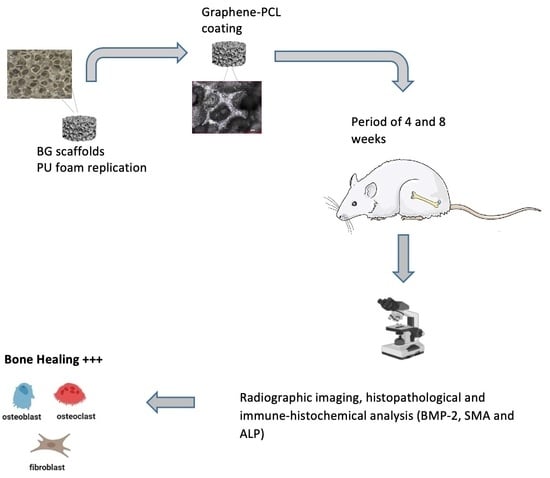
:1. Introduction
2. Experimental Studies
2.1. Materials
2.2. Scaffold Preparation
2.3. In Vivo Experiments
2.3.1. Rat Segmental Defect Model
2.3.2. Histopathological Method
2.3.3. Immunohistochemistry Method
2.3.4. Statistical Analysis
3. Results
3.1. Bioactive Glass Scaffolds
3.2. Histopathological Evaluation
3.3. Immunohistochemical Findings
3.3.1. BMP-2 Immunohistochemistry Results
3.3.2. Smooth Muscle Actin Immunohistochemistry Results
3.3.3. ALP Immunohistochemistry Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liu, X.; Rahaman, M.N.; Liu, Y.; Bal, B.S.; Bonewald, L.F. Enhanced Bone Regeneration in Rat Calvarial Defects Implanted with Surface-Modified and BMP-Loaded Bioactive Glass (13–93) Scaffolds. Acta Biomater. 2013, 9, 7506–7517. [Google Scholar] [CrossRef] [PubMed]
- Brydone, A.S.; Meek, D.; MacLaine, S. Bone Grafting, Orthopaedic Biomaterials, and the Clinical Need for Bone Engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The Story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Miron, R.; Zhao, Y.; Zhang, Q.; Wu, C.; Geng, S. In Vivo Experimental Study on Bone Regeneration in Critical Bone Defects Using PIB Nanogels/Boron-Containing Mesoporous Bioactive Glass Composite Scaffold. Int. J. Nanomed. 2015, 10, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O. Graphene Scaffolds in Progressive Nanotechnology/Stem Cell-Based Tissue Engineering of the Nervous System. J. Mater. Chem. B 2016, 4, 3169–3190. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liu, H.; Lin, X.; Ma, L.; Zheng, X.; Liu, Y.; Huang, P.; Yu, S.; Zhang, W.; Lin, M.; et al. 3D Interpenetrated Graphene Foam/58S Bioactive Glass Scaffolds for Electrical-Stimulation-Assisted Differentiation of Rabbit Mesenchymal Stem Cells to Enhance Bone Regeneration. J. Biomed. Nanotechnol. 2019, 15, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Ma, W.; Zhou, Y.; Gui, Z.; Yao, A.; Wang, D.; Takemura, A.; Uemura, M.; Lin, K.; Xu, Y. Stimulatory Effects of Boron Containing Bioactive Glass on Osteogenesis and Angiogenesis of Polycaprolactone: In Vitro Study. BioMed Res. Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Yang, C.; Gao, Y.; Wang, C.; Li, M.; Guo, H.; Tong, Q. Fabrication and Characterization of Strontium-Doped Borate-Based Bioactive Glass Scaffolds for Bone Tissue Engineering. J. Alloys Compd. 2018, 743, 564–569. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, W.; Rahaman, M.N.; Day, D.E. Bone Regeneration in Rat Calvarial Defects Implanted with Fibrous Scaffolds Composed of a Mixture of Silicate and Borate Bioactive Glasses. Acta Biomater. 2013, 9, 9126–9136. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Rahaman, M.N.; Fu, H.; Liu, X. Silicate, Borosilicate, and Borate Bioactive Glass Scaffolds with Controllable Degradation Rate for Bone Tissue Engineering Applications. I. Preparation and in Vitro Degradation. J. Biomed. Mater. Res. Part A 2010, 95, 164–171. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and Bioactive Porous Polymer/Inorganic Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Türk, M.; Deliormanlı, A.M. Electrically Conductive Borate-Based Bioactive Glass Scaffolds for Bone Tissue Engineering Applications. J. Biomater. Appl. 2017, 32, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Deliormanlı, A.M. Fabrication and Characterization of Poly (ε-Caprolactone) Coated Silicate and Borate based Bioactive Glass Scaffolds. J. Compos. Mater. 2016, 50, 917–928. [Google Scholar] [CrossRef]
- Türk, M.; Deliormanlı, A.M. Graphene-Containing PCL- Coated Porous 13-93B3 Bioactive Glass Scaffolds for Bone Regeneration. Mater. Res. Express 2018, 5, 45406. [Google Scholar] [CrossRef]
- Deliormanlı, A.M.; Türk, M.; Atmaca, H. Response of Mouse Bone Marrow Mesenchymal Stem Cells to Graphene-containing Grid-like Bioactive Glass Scaffolds Produced by Robocasting. J. Biomater. Appl. 2018, 33, 488–500. [Google Scholar] [CrossRef]
- Qi, X.; Wang, H.; Zhang, Y.; Pang, L.; Xiao, W.; Jia, W.; Zhao, S.; Wang, D.; Huang, W.; Wang, Q. Mesoporous Bioactive Glass-Coated 3D Printed Borosilicate Bioactive Glass Scaffolds for Improving Repair of Bone Defects. Int. J. Biol. Sci. 2018, 14, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liu, T.; Shuai, C.; Peng, S. Enhancement Mechanisms of Graphene in Nano-58S Bioactive Glass Scaffold: Mechanical and Biological Performance. Sci. Rep. 2014, 4, 4712. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Gao, X.; Shi, X.L.; Lu, S.; Yang, W.; Duan, C.; Chen, Z.G. Graphene Oxide and Adiponectin-Functionalized Sulfonated Poly(Etheretherketone) with Effective Osteogenicity and Remotely Repeatable Photodisinfection. Chem. Mater. 2020, 32, 2180–2193. [Google Scholar] [CrossRef]
- Prasadh, S.; Suresh, S.; Wong, R. Osteogenic Potential of Graphene in Bone Tissue Engineering Scaffolds. Materials 2018, 11, 1430. [Google Scholar] [CrossRef] [PubMed]
- Basal, O.; Ozmen, O.; Deliormanlı, A.M. Effect of Polycaprolactone Scaffolds Containing Different Weights of Graphene on Healing in Large Osteochondral Defect Model. J. Biomater. Sci. Polym. Ed. 2022, 33, 1123–1139. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.S.; Fernandes, A.B.; Maciel, J.V.; Netto, J.D.N.; Bolognese, A.M. New Methodology for Evaluating Osteoclastic Activity Induced by Orthodontic Load. J. Appl. Oral Sci. 2015, 23, 19–25. [Google Scholar] [CrossRef]
- Ouis, M.A.; Abdelghany, A.M.; ElBatal, H.A. Corrosion Mechanism and Bioactivity of Borate Glasses Analogue to Hench’s Bioglass. Process. Appl. Ceram. 2012, 6, 141–149. [Google Scholar] [CrossRef]
- Kim, T.; Lee, S.; Park, S.-Y.; Chung, I. Biodegradable PCL-b-PLA Microspheres with Nanopores Prepared via RAFT Polymerization and UV Photodegradation of Poly (Methyl Vinyl Ketone) Blocks. Polymers 2021, 13, 3964. [Google Scholar] [CrossRef]
- Ruiz, S.; Tamayo, J.A.; Delgado Ospina, J.; Navia Porras, D.P.; Valencia Zapata, M.E.; Mina Hernandez, J.H.; Valencia, C.H.; Zuluaga, F.; Grande Tovar, C.D. Antimicrobial Films Based on Nanocomposites of Chitosan/Poly (Vinyl Alcohol)/Graphene Oxide for Biomedical Applications. Biomolecules 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Liu, X.; Bal, B.S.; Day, D.E.; Bi, L.; Bonewald, L.F. Bioactive Glass in Bone Tissue Engineering. Ceram. Trans. 2012, 237, 73–82. [Google Scholar] [CrossRef]
- Shi, J.; Fang, Y. Biomedical Applications of Graphene. Graphene Fabr. Charact. Prop. Appl. 2017, 2, 215–232. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Du, D.; Lin, Y. Graphene Based Materials for Biomedical Applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Zeimaran, E.; Pourshahrestani, S.; Nam, H.Y.; Razak, N.A.B.A.; Kalantari, K.; Kamarul, T.; Salamatinia, B.; Kadri, N.A. Engineering Stiffness in Highly Porous Biomimetic Gelatin/Tertiary Bioactive Glass Hybrid Scaffolds Using Graphene Nanosheets. React. Funct. Polym. 2020, 154, 104668. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, J.; Liu, F.; Nie, Y.; Ren, L.; Liu, B. A New Composite Scaffold of Bioactive Glass Nanoparticles/Graphene: Synchronous Improvements of Cytocompatibility and Mechanical Property. Colloids Surf. B Biointerfaces 2016, 145, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Jung, S.; Day, D.; Neidig, K.; Dusevich, V.; Eick, D.; Bonewald, L. Evaluation of Bone Regeneration, Angiogenesis, and Hydroxyapatite Conversion in Critical-Sized Rat Calvarial Defects Implanted with Bioactive Glass Scaffolds. J. Biomed. Mater. Res. Part A 2012, 100, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wan, B.; Wang, R.; Zhang, B.; Luo, P.; Wang, D.; Nie, J.-J.; Chen, D.; Wu, X. Mechanical Stimulation on Mesenchymal Stem Cells and Surrounding Microenvironments in Bone Regeneration: Regulations and Applications. Front. Cell Dev. Biol. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.R.; Szczodry, M.; Bruno, S. Animal Models for Cartilage Regeneration and Repair. Tissue Eng. Part B Rev. 2010, 16, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Deliormanlı, A.M. Direct Write Assembly of Graphene/Poly(ε-Caprolactone) Composite Scaffolds and Evaluation of Their Biological Performance Using Mouse Bone Marrow Mesenchymal Stem Cells. Appl. Biochem. Biotechnol. 2019, 188, 1117–1133. [Google Scholar] [CrossRef]
- Ren, Q.; Cai, M.; Zhang, K.; Ren, W.; Su, Z.; Yang, T.; Sun, T.; Wang, J. Effects of Bone Morphogenetic Protein-2 (BMP-2) and Vascular Endothelial Growth Factor (VEGF) Release from Polylactide-Poly (Ethylene Glycol)-Polylactide (PELA) Microcapsule-Based Scaffolds on Bone. Braz. J. Med. Biol. Res. 2017, 51, 0809050139. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-J.; Peng, W.-X.; Zhang, M.-B. LncRNA KCNQ1OT1 promotes osteogenic differentiation via miR-205-5p/RICTOR axis. Exp. Cell Res. 2022, 415, 113119. [Google Scholar] [CrossRef] [PubMed]
- Basal, O.; Atay, T.; Ciris, I.M.; Baykal, Y.B. Epidermal Growth Factor (EGF) Promotes Bone Healing in Surgically Induced Osteonecrosis of the Femoral Head (ONFH). Bosn. J. Basic Med. Sci. 2018, 18, 352–360. [Google Scholar] [CrossRef]
- Guan, H.; Kong, N.; Tian, R.; Cao, R.; Liu, G.; Li, Y.; Wei, Q.; Jiao, M.; Lei, Y.; Xing, F.; et al. Melatonin increases bone mass in normal, perimenopausal, and postmenopausal osteoporotic rats via the promotion of osteogenesis. J. Transl. Med. 2022, 20, 1–15. [Google Scholar] [CrossRef]
- Koosha, E.; Eames, B.F. Two Modulators of Skeletal Development: BMPs and Proteoglycans. J. Dev. Biol. 2022, 10, 15. [Google Scholar] [CrossRef]
- Park, K.O.; Lee, J.H.; Park, J.H.; Shin, Y.C.; Huh, J.B.; Bae, J.H.; Kang, S.H.; Hong, S.W.; Kim, B.; Yang, D.J.; et al. Graphene Oxide-Coated Guided Bone Regeneration Membranes with Enhanced Osteogenesis: Spectroscopic Analysis and Animal Study. Appl. Spectrosc. Rev. 2016, 51, 540–551. [Google Scholar] [CrossRef]
- Lim, J.; Lee, J.; Yun, H.S.; Shin, H.I.; Park, E.K. Comparison of Bone Regeneration Rate in Flat and Long Bone Defects: Calvarial and Tibial Bone. Tissue Eng. Regen. Med. 2013, 10, 336–340. [Google Scholar] [CrossRef]












| 4 Weeks | 8 Weeks | ||
|---|---|---|---|
| New bone area (mm2) | Control | 2.66 ± 0.38 a | 5.28 ± 0.61 a |
| Group 1 | 19.40 ± 2.07 b | 23.98 ± 0.91 b | |
| Group 2 | 20.24 ± 1.37 c | 31.18 ± 1.09 c | |
| Group 3 | 25.20 ± 1.48 d | 36.00 ± 3.36 d | |
| Group 4 | 28.00 ± 1.73 e | 41.26 ± 0.71 e | |
| Residual material area (mm2) | Control | 15.70 ± 1.78 a | 7.84 ± 0.92 a |
| Group 1 | 19.40 ± 1.14 b | 8.48 ± 0.91 a | |
| Group 2 | 18.20 ± 2.28 a | 7.48 ± 1.58 a | |
| Group 3 | 22.20 ± 1.92 c | 10.12 ± 1.19 b | |
| Group 4 | 25.40 ± 2.07 d | 12.80 ± 1.92 c | |
| Osteoclast number | Control | 10.40 ± 0.54 a | 14.40 ± 1.14 a |
| Group 1 | 13.80 ± 1.48 b | 18.20 ± 1.64 b | |
| Group 2 | 19.00 ± 1.87 c | 20.00 ± 1.58 b | |
| Group 3 | 20.00 ± 1.58 c | 24.00 ± 1.58 c | |
| Group 4 | 24.00 ± 1.58 d | 26.60 ± 2.07 d | |
| Osteoblast number | Control | 11.00 ± 1.58 a | 20.20 ± 2.28 a |
| Group 1 | 16.20 ± 1.48 b | 25.00 ± 1.87 b | |
| Group 2 | 17.40 ± 1.14 b | 27.60 ± 1.14 c | |
| Group 3 | 19.60 ± 0.89 c | 28.40 ± 2.96 c | |
| Group 4 | 23.60 ± 1.34 d | 35.20 ± 0.83 d |
| BMP-2 | SMA | ALP | |
|---|---|---|---|
| 4-week | |||
| Control | 1.20 ± 0.44 a | 0.80 ± 0.44 a | 1.40 ± 0.54 a |
| Group 1 | 2.60 ± 0.54 b | 1.40 ± 0.54 a | 2.20 ± 0.83 b |
| Group 2 | 2.40 ± 0.54 b | 1.60 ± 0.89 a | 2.00 ± 0.70 b |
| Group 3 | 2.40 ± 0.54 b | 1.40 ± 0.54 a | 1.80 ± 0.83 b |
| Group 4 | 2.60 ± 0.54 b | 1.80 ± 0.83 b | 2.00 ± 1.00 b |
| 8-week | |||
| Control | 1.00 ± 0.00 a | 1.40 ± 0.54 a | 1.60 ± 0.54 a |
| Group 1 | 1.75 ± 0.50 a | 2.20 ± 0.83 b | 2.00 ± 0.70 b |
| Group 2 | 2.00 ± 0.00 b | 2.20 ± 0.44 b | 2.20 ± 0.44 b |
| Group 3 | 2.00 ± 0.81 b | 2.40 ± 0.54 b | 2.60 ± 0.54 b |
| Group 4 | 2.75 ± 0.50 b | 2.40 ± 0.89 b | 2.80 ± 0.44 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basal, O.; Ozmen, O.; Deliormanlı, A.M. Bone Healing in Rat Segmental Femur Defects with Graphene-PCL-Coated Borate-Based Bioactive Glass Scaffolds. Polymers 2022, 14, 3898. https://doi.org/10.3390/polym14183898
Basal O, Ozmen O, Deliormanlı AM. Bone Healing in Rat Segmental Femur Defects with Graphene-PCL-Coated Borate-Based Bioactive Glass Scaffolds. Polymers. 2022; 14(18):3898. https://doi.org/10.3390/polym14183898
Chicago/Turabian StyleBasal, Ozgur, Ozlem Ozmen, and Aylin M. Deliormanlı. 2022. "Bone Healing in Rat Segmental Femur Defects with Graphene-PCL-Coated Borate-Based Bioactive Glass Scaffolds" Polymers 14, no. 18: 3898. https://doi.org/10.3390/polym14183898
APA StyleBasal, O., Ozmen, O., & Deliormanlı, A. M. (2022). Bone Healing in Rat Segmental Femur Defects with Graphene-PCL-Coated Borate-Based Bioactive Glass Scaffolds. Polymers, 14(18), 3898. https://doi.org/10.3390/polym14183898