Properties and Hydrolysis Behavior of Celluloses of Different Origin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cellulose Samples
2.1.1. Synthesis of BC
2.1.2. Synthesis of Synthetic Cellulose
2.1.3. Isolation of Plant-Based Cellulose
2.2. Analysis of Chemical Composition and Degree of Polymerization of Cellulose Samples
2.2.1. X-ray Diffraction Analysis of Cellulose Samples
2.2.2. Specific Surface and Pore Volume of Cellulose Samples
2.2.3. Infrared Spectroscopy
2.2.4. Scanning Electron Microscopy
2.3. Enzymatic Hydrolysis
3. Results and Discussion
3.1. Appearance and Properties of Cellulose Samples
3.2. Infrared Spectroscopy
3.3. Scanning Electron Microscopy (SEM)
3.4. Enzymatic Hydrolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Imai, T.; Yui, T.; Yao, M.; Saxena, I. Cellulose-synthesizing machinery in bacteria. Cellulose 2021, 29, 2755–2777. [Google Scholar] [CrossRef]
- Klemm, D.; Petzold-Welcke, K.; Kramer, F.; Richter, T.; Raddatz, V.; Fried, W.; Nietzsche, S.; Bellmann, T.; Fischer, D. Biotech Nanocellulose: A review on progress in product design and today’s state of technical and medical applications. Carbohydr. Polym. 2020, 254, 117313. [Google Scholar] [CrossRef] [PubMed]
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.R.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial cellulose: A smart biomaterial with diverse applications. Mater. Sci. Eng. A 2021, 145, 100623. [Google Scholar] [CrossRef]
- Wahid, F.; Huang, L.H.; Zhao, X.Q.; Li, W.C.; Wang, Y.Y.; Jia, S.R.; Zhong, C. Bacterial cellulose and its potential for biomedical applications. Biotechnol. Adv. 2021, 53, 107856. [Google Scholar] [CrossRef]
- Wang, L.; Mao, L.; Qi, F.; Li, X.; Ullah, M.W.; Zhao, M.; Shi, Z.; Yang, G. Synergistic effect of highly aligned bacterial cellulose/gelatin membranes and electrical stimulation on directional cell migration for accelerated wound healing. Chem. Eng. J. 2021, 424, 130563. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Subhan, F.; Islam, S.U.; Khan, S.; Shah, N.; Manan, S.; Ullah, M.W.; Yang, G. Development of three-dimensional bacterial cellulose/chitosan scaffolds: Analysis of cell-scaffold interaction for potential application in the diagnosis of ovarian cancer. Int. J. Biol. Macromol. 2019, 137, 1050–1059. [Google Scholar] [CrossRef]
- Farooq, U.; Ullah, M.W.; Yang, Q.; Aziz, A.; Xu, J.; Zhou, L.; Wang, S. High-density phage particles immobilization in surface-modified bacterial cellulose for ultra-sensitive and selective electrochemical detection of Staphylococcus aureus. Biosens. Bioelectron. 2020, 157, 112163. [Google Scholar] [CrossRef]
- Shoukat, A.; Wahid, F.; Khan, T.; Siddique, M.; Nasreen, S.; Yang, G.; Ullah, M.W.; Khan, R. Titanium oxide-bacterial cellulose bioadsorbent for the removal of lead ions from aqueous solution. Int. J. Biol. Macromol. 2019, 129, 965–971. [Google Scholar] [CrossRef]
- Sheng, N.; Zhang, M.; Song, Q.; Zhang, H.; Chen, S.; Wang, H.; Zhang, K. Enhanced salinity gradient energy harvesting with oppositely charged bacterial cellulose-based composite membranes. Nano Energy 2022, 101, 107548. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Park, J.K. Bacterial cellulose–poly (3, 4-ethylenedioxythiophene)–poly (styrenesulfonate) composites for optoelectronic applications. Carbohydr. Polym. 2015, 127, 86–93. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.R.; Ullah, M.W.; Booth, P.; Pei, E.; Yang, G. The use of bacterial polysaccharides in bioprinting. Biotechnol. Adv. 2019, 37, 107448. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.W.; Ul-Islam, M.; Khan, S.; Kim, Y.; Park, J.K. Innovative production of bio cellulose using a cell-free system derived from a single cell line. Carbohydr. Polym. 2015, 132, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ullah, M.W.; Ul-Islam, M.; Khan, S.; Jang, J.H.; Park, J.K. Self-assembly of bio-cellulose nanofibrils through intermediate phase in a cell-free enzyme system. Biochem. Eng. J. 2019, 142, 135–144. [Google Scholar] [CrossRef]
- Nidetzky, B.; Zhong, C. Phosphorylase-catalyzed bottom-up synthesis of short-chain soluble cello-oligosaccharides and property-tunable cellulosic materials. Biotechnol. Adv. 2021, 51, 107633. [Google Scholar] [CrossRef]
- Ioelovich, M.; Morag, E. Effect of cellulose structure on enzymatic hydrolysis. Bioresources 2011, 6, 2818–2835. [Google Scholar] [CrossRef]
- Rohrbach, J.C.; Luterbacher, J.S. Investigating the effects of substrate morphology and experimental conditions on the enzymatic hydrolysis of lignocellulosic biomass through modeling. Biotechnol. Biofuels Bioprod. 2021, 14, 103. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, J.; Nawaz, A.; ul Haq, I.; Zhou, X.; Xu, Y. Comprehensive investigation of multiples factors in sulfuric acid pretreatment on the enzymatic hydrolysis of waste straw cellulose. Bioresour. Technol. 2021, 340, 125740. [Google Scholar] [CrossRef]
- Skiba, E.A.; Budaeva, V.V.; Baibakova, O.V.; Zolotukhin, V.N.; Sakovich, G.V. Dilute nitric-acid pretreatment of oat hulls for ethanol production. Biochem. Eng. J. 2017, 126, 118–125. [Google Scholar] [CrossRef]
- Hallac, B.B.; Ragauskas, A.J. Analyzing cellulose degree of polymerization and its relevancy to cellulosic ethanol. Biofuels Bioprod. Biorefining 2011, 5, 215–225. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A critical review on analysis in pretreatment of lignocelluloses. Bioresour. Technol. 2016, 203, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yi, Z.; Huang, J.; Li, F.; Hao, B.; Li, M.; Hong, S.; Lv, Y.; Sun, W.; Ragauskas, A.; et al. Three lignocellulose features that distinctively affect biomass enzymatic digestibility under NaOH and H2SO4 pretreatments in Miscanthus. Bioresour. Technol. 2013, 130, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Ragauskas, A. Pretreatment and lignocellulosic chemistry. BioEnergy Res. 2012, 5, 1043–1066. [Google Scholar] [CrossRef]
- Wu, S.; Jiang, X.; Jiang, H.; Wu, S.; Ding, S.; Jin, Y. Impacts of cotton linter pulp characteristics on the processivity of glycoside hydrolase family 5 endoglucanase from Volvariella Volvacea. Cellulose 2021, 28, 1947–1959. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, J.; Zhang, X.; Tan, T. The correlation between cellulose allomorphs (I and II) and conversion after removal of hemicellulose and lignin of lignocellulose. Bioresour. Technol. 2015, 193, 164–170. [Google Scholar] [CrossRef]
- Gomide, F.T.F.; da Silva, A.S.; da Silva Bon, E.P.; Alves, T.L.M. Modification of microcrystalline cellulose structural properties by ball-milling and ionic liquid treatments and their correlation to enzymatic hydrolysis rate and yield. Cellulose 2019, 26, 7323–7335. [Google Scholar] [CrossRef]
- Goh, W.N.; Rosma, A.; Kaur, B.; Fazilah, A.; Karim, A.A.; Rajeev, B. Fermentation of black tea broth (Kombucha): I. Effects of sucrose concentration and fermentation time on the yield of microbial cellulose. Int. Food Res. J. 2012, 19, 109–117. [Google Scholar]
- Budaeva, V.V.; Makarova, E.I.; Gismatulina, Y.A. Integrated flowsheet for conversion of nonwoody biomass into polyfunctional materials. Key Eng. Mater. 2016, 670, 202–206. [Google Scholar] [CrossRef]
- Kashcheyeva, E.I.; Gismatulina, Y.A.; Budaeva, V.V. Pretreatments of Non-Woody Cellulosic Feedstocks for Bacterial Cellulose Synthesis. Polymers 2019, 11, 1645. [Google Scholar] [CrossRef]
- Gladysheva, E.K.; Skiba, E.A.; Zolotukhin, V.N.; Sakovich, G.V. Study of the Conditions for the Biosynthesis of Bacterial Cellulose by the Producer Medusomyces gisevii Sa-12. Appl. Biochem. Microbiol. 2018, 54, 179–187. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Budaeva, V.V.; Sakovich, G.V.; Vasilyeva, O.Y.; Zueva, G.A.; Gusar, A.S.; Dorogina, O.V. Features of the resource species Miscanthus sacchariflorus (Maxim.) Hack. when introduced in West Siberia. Vavilovskii Zhurnal Genet. I Sel. = Vavilov J. Genet. Breed. 2019, 23, 933–940. [Google Scholar] [CrossRef]
- Obolenskaya, A.V.; Yelnitskaya, Z.P.; Leonovich, A.A. Laboratory Works on Wood and Cellulose Chemistry: Textbook for Higher Educational Institutions; Laboratornye Raboty Po Khimii Drevesiny I Tsellyulozy; Ecology Publisher: Moscow, Russia, 1991; p. 320. [Google Scholar]
- Technical Association of the Pulp and Paper Industry. TAPPI T 222 om-83 Standard: Acid-Insoluble Lignin in Wood and Pulp, Specifications of the Technical Association of the Pulp and Paper Industry. Atlanta, GA, USA. 1988. Available online: https://standards.globalspec.com/std/10402544/tappi-t-222 (accessed on 1 October 2021).
- Technical Association of the Pulp and Paper Industry. TAPPI T 211 om-85 standard: Ash in wood, pulp, paper, and paperboard, specifications of the Technical Association of the Pulp and Paper Industry. Atlanta, GA, USA. 1985. Available online: https://standards.globalspec.com/std/1524019/TAPPI%20T%20211 (accessed on 1 October 2021).
- Bogolitsyn, K.; Parshina, A.; Aleshina, L. Structural features of brown algae cellulose. Cellulose 2020, 27, 9787–9800. [Google Scholar] [CrossRef]
- Torlopov, M.A.; Mikhaylov, V.I.; Udoratina, E.V.; Aleshina, L.A.; Prusskii, A.I.; Tsvetkov, N.V.; Krivoshapkin, P.V. Cellulose nanocrystals with different length-to-diameter ratios extracted from various plants using novel system acetic acid/phosphotungstic acid/octanol-1. Cellulose 2018, 25, 1031–1046. [Google Scholar] [CrossRef]
- French, A.D. Increment in evolution of cellulose crystallinity analysis. Cellulose 2020, 27, 5445–5448. [Google Scholar] [CrossRef]
- Terinte, N.; Ibbett, R.; Schuster, K.C. Overview on native cellulose and microcrystalline cellulose I structure studied by X-ray diffraction (WAXD): Comparison between measurement techniques. Lenzing. Ber. 2011, 89, 118–131. [Google Scholar]
- Ahvenainen, P.; Kontro, I.; Svedström, K. Comparison of sample crystallinity determination methods by X-ray diffraction for challenging cellulose I materials. Cellulose 2016, 23, 1073–1086. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Mironova, G.F.; Skiba, E.A.; Kukhlenko, A.A. Preparing Nutrient Media from Lignocellulose: Optimizing the Composition of a Multienzyme Compound. Catal. Ind. 2020, 12, 162–168. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Van der Cruijsen, K.; Hassan, M.A.; van Erven, G.; Dolstra, O.; Trindade, L.M. Breeding Targets to Improve Biomass Quality in Miscanthus. Molecules 2021, 26, 254. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kong, Y.; Hu, R.; Zhou, G. Miscanthus: A fast-growing crop for environmental remediation and biofuel production. GCB Bioenergy 2021, 13, 58–69. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Kassab, Z.; Kassem, I.; Hannache, H.; Bouhfid, R.; Qaiss, A.E.K.; Achaby, M.E. Tomato plant residue as new renewable source for cellulose production: Extraction of cellulose nanocrystals with different surface functionalities. Cellulose 2020, 27, 4287–4303. [Google Scholar] [CrossRef]
- Ait Benhamou, A.; Kassab, Z.; Nadifiyine, M.; Salim, M.H.; Sehaqui, H.; Moubarik, A.; Achaby, M.E. Extraction, characterization and chemical functionalization of phosphorylated cellulose derivatives from Giant Reed Plant. Cellulose 2021, 28, 4625–4642. [Google Scholar] [CrossRef]
- Gabriel, T.; Wondu, K.; Dilebo, J. Valorization of khat (Catha edulis) waste for the production of cellulose fibers and nanocrystals. PLoS ONE 2021, 16, e0246794. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, J.; Huang, T.; Bian, H.; Wang, R.; Sha, J.; Dai, H. Near-complete enzymatic hydrolysis efficiency of Miscanthus using hydrotropic fractionation at atmospheric pressure. Ind. Crops Prod. 2020, 149, 112365. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, X.; Yang, J.; Lin, Q.; Wang, J.; Zhu, Q. Comparison of succinylation methods for bacterial cellulose and adsorption capacities of bacterial cellulose derivatives for Cu2+ ion. Polym. Bull. 2011, 67, 401–412. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Lenz, J.; Esterbauer, H.; Sattler, W.; Schurz, J.; Wrentschur, E. Changes of structure and morphology of regenerated cellulose caused by acid and enzymatic hydrolysis. J. Appl. Polym. Sci. 1990, 41, 1315–1326. [Google Scholar] [CrossRef]
- Schurz, J.; Billiani, J.; Hönel, A.; Eigner, W.D.; Jánosi, A.; Hayn, M.; Esterbauer, H. Reaktionsmechanismus und Strukturänderungen beim enzymatischen Abbau von Cellulose durch Trichoderma-reesei-Cellulase. Acta Polym. 1985, 36, 76–80. [Google Scholar] [CrossRef]
- Schurz, J.; Zipper, P.; Lenz, J. Structural studies on polymers as prerequisites for degradation. J. Macromol. Sci. Part A Pure Appl. Chem. 1993, 30, 603–619. [Google Scholar] [CrossRef]
- Vaaje-Kolstad, G.; Westereng, B.; Horn, S.J.; Liu, Z.; Zhai, H.; Sørlie, M.; Eijsink, V.G. An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 2010, 330, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Westereng, B.; Ishida, T.; Vaaje-Kolstad, G.; Wu, M.; Eijsink, V.G.; Igarashi, K.; Sandgren, M. The putative endoglucanase PcGH61D from Phanerochaete chrysosporium is a metal-dependent oxidative enzyme that cleaves cellulose. PLoS ONE 2011, 6, e27807. [Google Scholar] [CrossRef] [PubMed]
- Ul-Islam, M.; Khan, S.; Ullah, M.W.; Park, J.K. Comparative study of plant and bacterial cellulose pellicles regenerated from dissolved states. Int. J. Biol. Macromol. 2019, 137, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Bt Hj Idrus, R.; Fauzi, M.B. Plant- vs. Bacterial-Derived Cellulose for Wound Healing: A Review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Zhuang, X.; Chen, H.; Qin, Y.; Cao, J.; Fan, F.; Lan, T. Effect of supramolecular structural changes during the crystalline transformation of cellulose on its enzymatic hydrolysis. Ind. Crops Prod. 2022, 180, 114687. [Google Scholar] [CrossRef]
- Jeoh, T.; Cardona, M.J.; Karuna, N.; Mudinoor, A.R.; Nill, J. Mechanistic kinetic models of enzymatic cellulose hydrolysis—A review. Biotechnol. Bioeng. 2017, 114, 1369–1385. [Google Scholar] [CrossRef]




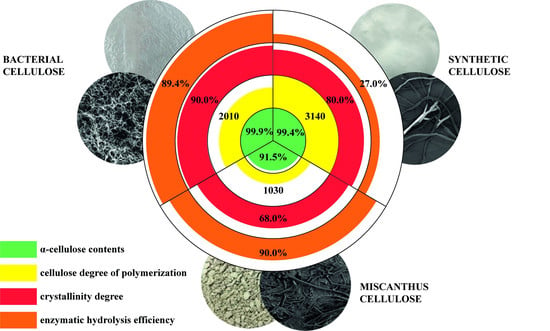
| Characteristics | Cellulose Sample | ||
|---|---|---|---|
| BC | Synthetic Cellulose | Miscanthus Cellulose | |
| Contents, % α-cellulose lignin ash pentosans | 99.9 ± 0.4 0 ± 0.1 0 ± 0.01 0 ± 0.1 | 99.4 ± 0.4 0 ± 0.1 0 ± 0.01 0 ± 0.1 | 91.5 ± 0.4 1.4 ± 0.1 0.7 ± 0.01 6.4 ± 0.1 |
| Degree of polymerization | 2010 ± 10 | 3140 ± 10 | 1030 ± 10 |
| Degree of crystallinity, % | 90 ± 5 | 80 ± 5 | 68 ± 5 |
| Specific surface, m2/g | 5.265 | 3.012 | 1.278 |
| Pore volume, cm3/g | 0.028 | 0.016 | 0.008 |
| Absorption Band Assignment * | Absorption Band Peak, cm−1 | ||
|---|---|---|---|
| a | b | c | |
| ν OH groups | 3424 | 3435 | 3424 |
| ν CH, CH2 groups | 2918 | 2901 | 2916 |
| δ OH groups of tightly bound water | 1654 | 1637 | 1632 |
| δ CH2, CH groups | 1430 1373 | 1431 1372 | 1435 1373 |
| ν C-O bonds (bands typical of polysaccharides due to present C-O-C acetyl bonds and C-O bonds in alcohols) | 1160 1110 1059 | 1165 1114 1058 | 1165 1112 1058 |
| β-1,4 bonds | 899 | 898 | 897 |
| Substrate | C0i, (g/L) | ki, h−1 |
|---|---|---|
| BC | 29.9 | 0.0500 |
| Synthetic cellulose | 10.7 | 0.0215 |
| Miscanthus cellulose | 30.2 | 0.0716 |
| Sample | RS Concentration (g/L) | RS Yield (%) |
|---|---|---|
| BC | 29.8 ± 0.1 | 89.4 ± 1.4 |
| Synthetic cellulose | 9.0 ± 0.1 | 27.0 ± 1.4 |
| Miscanthus cellulose | 30.0 ± 0.1 | 90.0 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashcheyeva, E.I.; Gismatulina, Y.A.; Mironova, G.F.; Gladysheva, E.K.; Budaeva, V.V.; Skiba, E.A.; Zolotuhin, V.N.; Shavyrkina, N.A.; Kortusov, A.N.; Korchagina, A.A. Properties and Hydrolysis Behavior of Celluloses of Different Origin. Polymers 2022, 14, 3899. https://doi.org/10.3390/polym14183899
Kashcheyeva EI, Gismatulina YA, Mironova GF, Gladysheva EK, Budaeva VV, Skiba EA, Zolotuhin VN, Shavyrkina NA, Kortusov AN, Korchagina AA. Properties and Hydrolysis Behavior of Celluloses of Different Origin. Polymers. 2022; 14(18):3899. https://doi.org/10.3390/polym14183899
Chicago/Turabian StyleKashcheyeva, Ekaterina I., Yulia A. Gismatulina, Galina F. Mironova, Evgenia K. Gladysheva, Vera V. Budaeva, Ekaterina A. Skiba, Vladimir N. Zolotuhin, Nadezhda A. Shavyrkina, Aleksey N. Kortusov, and Anna A. Korchagina. 2022. "Properties and Hydrolysis Behavior of Celluloses of Different Origin" Polymers 14, no. 18: 3899. https://doi.org/10.3390/polym14183899
APA StyleKashcheyeva, E. I., Gismatulina, Y. A., Mironova, G. F., Gladysheva, E. K., Budaeva, V. V., Skiba, E. A., Zolotuhin, V. N., Shavyrkina, N. A., Kortusov, A. N., & Korchagina, A. A. (2022). Properties and Hydrolysis Behavior of Celluloses of Different Origin. Polymers, 14(18), 3899. https://doi.org/10.3390/polym14183899