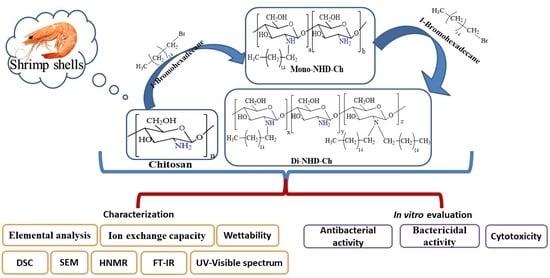
Preparation, Characterization, and Bio Evaluation of Fatty N- Hexadecanyl Chitosan Derivatives for Biomedical Applications
Abstract
:1. Introduction
2. Results
2.1. Characterization
2.2. Bio-Evaluation
2.2.1. Antibacterial Assay Using Agar-Well Diffusion Method
2.2.2. Determination of MIC
2.2.3. Bactericidal Behavior
2.2.4. Cytotoxicity
3. Materials and Methods
3.1. Materials
Microorganisms
3.2. Methods
3.2.1. Preparation of N-Alkyl Chitosan Derivative
3.2.2. Characterization of Prepared Materials
3.2.3. In-Vitro Evaluation
Antibacterial Evaluation Using Agar-Well Diffusion Method
Minimum Inhibitory Concentration (MIC) Determination
Bactericidal Activity
Cytotoxicity of Chitosan and Its Alkyl Derivatives
4. Conclusions
- Physicochemical characterization of the new derivatives, such as ion exchange capacity and water uptake, was significantly changed;
- The N-alkyl derivatives were confirmed by the shift of the polymer characterization peak in their electronic spectrum and the alky signals at the HNMR chart;
- An FT-IR analysis of the prepared derivatives exhibited a significant new peak;
- The thermal stability of the chitosan derivatives was enhanced in the new derivatives;
- The antibacterial evaluation demonstrated that this new N-Alkyl derivative of chitosan exhibited better antibacterial activity than chitosan.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, M.; Brooks, H.J.L.; Moratti, S.C.; Hanton, L.R.; Cabral, J.D. Reducing the Oxidation Level of Dextran Aldehyde in a Chitosan/Dextran-Based Surgical Hydrogel Increases Biocompatibility and Decreases Antimicrobial Efficacy. Int. J. Mol. Sci. 2015, 16, 13798–13814. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.R.; Abdel-Hay, F.I.; Tamer, T.M.; Ibrahim, E.M.A.-E.; Eldin, M.S.M. Antimicrobial activity of novel modified aminated chitosan with aromatic esters. Polym. Bull. 2019, 77, 1631–1647. [Google Scholar] [CrossRef]
- Omer, A.M.; Ammar, Y.; Mohamed, G.; ElBaky, Y.A.; Tamer, T.M. Preparation of Isatin/chitosan schiff base as novel antibacterial biomaterials. Egypt. J. Chem. 2019, 62, 123–131. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Liu, J. Chitosan-based nanoscale and non-nanoscale delivery systems for anticancer drugs: A review. Eur. Polym. J. 2021, 154, 110533. [Google Scholar] [CrossRef]
- Abdelrazik, K.V.T.M.; Aacute, K.; Mohyeldin, M.; Soltes, L. Free Radical Scavenger Activity of Cinnamyl Chitosan Schiff Base. J. Appl. Pharm. Sci. 2016, 6, 130–136. [Google Scholar] [CrossRef]
- Lestari, W.; Yusry, W.N.A.W.; Haris, M.S.; Jaswir, I.; Idrus, E. A glimpse on the function of chitosan as a dental hemostatic agent. Jpn. Dent. Sci. Rev. 2020, 56, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Mohy Eldin, M.S.; Omer, A.M.; Wassel, M.A.; Tamer, T.M.; Abd Elmonem, M.S.; Ibrahim, S.A. Novel smart pH-sensitive chitosan grafted alginate hydrogel microcapsules for oral protein delivery: I. preparation and characterization. Int. J. Pharm. Pharm. Sci. 2015, 7, 320–326. [Google Scholar]
- Tamer, T.M.; Hassan, M.A.; Valachová, K.; Omer, A.M.; El-Shafeey, M.E.; Eldin, M.S.M.; Šoltés, L. Enhancement of wound healing by chitosan/hyaluronan polyelectrolyte membrane loaded with glutathione: In vitro and in vivo evaluations. J. Biotechnol. 2020, 310, 103–113. [Google Scholar] [CrossRef]
- Hassan, N.; Hendy, A.; Ebrahim, A.; Tamer, T.M. Synthesis and evaluation of novel O-functionalized aminated chitosan derivatives as antibacterial, antioxidant and anticorrosion for 316L stainless steel in simulated body fluid. J. Saudi Chem. Soc. 2021, 25, 101368. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.-G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Zhao, L. Antibacterial activity and mechanism of chitosan with ultra high molecular weight. Carbohydr. Polym. 2016, 148, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Le-Vinh, B.; Le, N.-M.N.; Nazir, I.; Matuszczak, B.; Bernkop-Schnürch, A. Chitosan based micelle with zeta potential changing property for effective mucosal drug delivery. Int. J. Biol. Macromol. 2019, 133, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Mohy Eldin, M.S.; Omer, A.M.; Wassel, M.A.; Tamer, T.M.; Abd Elmonem, M.S.; Ibrahim, S.A. Novel smart pH-sensitive chitosan grafted alginate hydrogel microcapsules for oral protein delivery: II. evaluation of the swelling behavior. Int. J. Pharm. Pharm. Sci. 2015, 7, 331–337. [Google Scholar]
- Wang, Y.; Xie, M.; Ma, G.; Fang, Y.; Yang, W.; Ma, N.; Fang, D.; Hu, Q.; Pei, F. The antioxidant and antimicrobial activities of different phenolic acids grafted onto chitosan. Carbohydr. Polym. 2019, 225, 115238. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, E.; Tamer, T.; Omer, A.; Eldin, M.M. Development of novel chitosan schiff base derivatives for cationic dye removal: Methyl orange model. Desalination Water Treat. 2016, 57, 22632–22645. [Google Scholar] [CrossRef]
- Shebl, A.; Omer, A.; Tamer, T. Adsorption of cationic dye using novel O-amine functionalized chitosan Schiff base derivatives: Isotherm and kinetic studies. Desalination Water Treat. 2018, 130, 132–141. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Ammar, Y.A.; Tamer, T.M.; Omer, A.M.; Ali, A.A. Development of low-cost chitosan derivatives based on marine waste sources as oil adsorptive materials: I. Preparation and characterization. Desalination Water Treat. 2017, 72, 41–51. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 119984. [Google Scholar] [CrossRef]
- Tikhonov, V.E.; Stepnova, E.A.; Babak, V.G.; Yamskov, I.A.; Guerrero, J.P.; Jansson, H.B.; Llorca, L.V.L.; Salinas, J.; Gerasimenko, D.V.; Avdienko, I.D.; et al. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl)succinoyl/-derivatives. Carbohydr. Polym. 2006, 64, 66–72. [Google Scholar] [CrossRef]
- Sahariah, P.; Benediktssdóttir, B.E.; Hjálmarsdóttir, M.Á.; Sigurjonsson, O.E.; Sørensen, K.K.; Thygesen, M.B.; Másson, M. Impact of Chain Length on Antibacterial Activity and Hemocompatibility of Quaternary N-Alkyl and N,N-Dialkyl Chitosan Derivatives. Biomacromolecules 2015, 16, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K.; Mori, S.; Nishiyama, Y.; Harata, M. N-Alkylation of chitin and some characteristics of the novel derivatives. Polym. Bull. 2002, 48, 159–166. [Google Scholar] [CrossRef]
- Polnok, A.; Borchard, G.; Verhoef, J.; Sarisuta, N.; Junginger, H. Influence of methylation process on the degree of quaternization of N-trimethyl chitosan chloride. Eur. J. Pharm. Biopharm. 2003, 57, 77–83. [Google Scholar] [CrossRef]
- Han, X.; Zheng, Z.; Yu, C.; Deng, Y.; Ye, Q.; Niu, F.; Chen, Q.; Pan, W.; Wang, Y. Preparation, characterization and antibacterial activity of new ionized chitosan. Carbohydr. Polym. 2022, 290, 119490. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, T.; Bangde, P.; Dandekar, P.; Jain, R. Greener approach for synthesis of N,N,N-trimethyl chitosan (TMC) using ternary deep eutectic solvents (TDESs). Carbohydr. Res. 2020, 493, 108033. [Google Scholar] [CrossRef]
- Hassan, M.A.; Tamer, T.M.; Valachová, K.; Omer, A.M.; El-Shafeey, M.; Eldin, M.S.M.; Šoltés, L. Antioxidant and antibacterial polyelectrolyte wound dressing based on chitosan/hyaluronan/phosphatidylcholine dihydroquercetin. Int. J. Biol. Macromol. 2020, 166, 18–31. [Google Scholar] [CrossRef]
- Yue, L.; Zheng, M.; Wang, M.; Khan, I.M.; Wang, B.; Ma, X.; Peng, C.; Wang, Z.; Xia, W. A general strategy to synthesis chitosan oligosaccharide-O-Terpenol derivatives with antibacterial properties. Carbohydr. Res. 2021, 503, 108315. [Google Scholar] [CrossRef]
- Demetgül, C.; Serin, S. Synthesis and characterization of a new vic-dioxime derivative of chitosan and its transition metal complexes. Carbohydr. Polym. 2008, 72, 506–512. [Google Scholar] [CrossRef]
- Kumar, S.; Koh, J.; Kim, H.; Gupta, M.K.; Dutta, P.K. A new chitosan-thymine conjugate: Synthesis: Characterization and biological activity. Int. J. Biol. Macromol. 2012, 50, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Signini, R.; Filho, S.P.C. On the preparation and characterization of chitosan hydrochloride. Polym. Bull. 1999, 42, 159–166. [Google Scholar] [CrossRef]
- Kumar, S.; Nigam, N.; Ghosh, T.; Dutta, P.K.; Yadav, R.S.; Pandey, A.C. Preparation, characterization, and optical properties of a chitosan-anthraldehyde crosslinkable film. J. Appl. Polym. Sci. 2009, 115, 3056–3062. [Google Scholar] [CrossRef]
- Lal, S.; Arora, S.; Sharma, C. Synthesis, thermal and antimicrobial studies of some Schiff bases of chitosan. J. Therm. Anal. 2016, 124, 909–916. [Google Scholar] [CrossRef]
- Zawadzki, J.; Kaczmarek, H. Thermal treatment of chitosan in various conditions. Carbohydr. Polym. 2010, 80, 394–400. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR studies of chitosan blends Thermochim. Acta 2003, 396, 153–166. [Google Scholar]
- Hamed, A.A.; Abdelhamid, I.A.; Saad, G.R.; Elkady, N.A.; Elsabee, M.Z. Synthesis, characterization and antimicrobial activity of a novel chitosan Schiff bases based on heterocyclic moieties. Int. J. Biol. Macromol. 2020, 153, 492–501. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Sinha, D.; Tiwari, A.K.; Singh, S.; Shukla, G.; Mishra, P.; Chandra, H.; Mishra, A.K. Synthesis, characterization and biological activity of Schiff base analogues of indole-3-carboxaldehyde. Eur. J. Med. Chem. 2008, 43, 160–165. [Google Scholar] [CrossRef]
- Gurkok, G.; Altanlar, N.; Suzen, S. Investigation of Antimicrobial Activities of Indole-3-Aldehyde Hydrazide/Hydrazone Derivatives. Chemotherapy 2008, 55, 15–19. [Google Scholar] [CrossRef]
- Yang, T.-C.; Chou, C.-C.; Li, C.-F. Antibacterial activity of N-alkylated disaccharide chitosan derivatives. Int. J. Food Microbiol. 2005, 97, 237–245. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.I.; Rogge, T.M.; Stevens, C.V.; Steurbaut, W.; Höfte, M.; Smagghe, G. Enhancement of fungicidal and insecticidal activity by reductive alkylation of chitosan. Pest Manag. Sci. 2006, 62, 890–897. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P.; Saesoo, S. Synthesis and antibacterial activity of methylated N-(4-N,N-dimethylaminocinnamyl) chitosan chloride. Eur. Polym. J. 2009, 45, 2319–2328. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Fahmy, M.M. Synthesis and Antimicrobial Activity of Some Novel Cross-Linked Chitosan Hydrogels. Int. J. Mol. Sci. 2012, 13, 11194–11209. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, F.; Yang, R. Synthesis and characterization of chitosan derivatives with dual-antibacterial functional groups. Int. J. Biol. Macromol. 2015, 75, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.R.; Fekry, A. Antimicrobial and anticorrosive activity of adsorbents based on chitosan Schiff’s base. Int. J. Electrochem. Sci. 2011, 6, 2488–2508. [Google Scholar]
- Hassan, M.A.; Tamer, T.M.; Rageh, A.A.; Abou-Zeid Alaa, M.; Abd El-Zaher, E.H.F.; Kenawy, E.-R. Insight into multidrug-resistant microorganisms from microbial infected diabetic foot ulcers. Diabetes Metab. Syndr. 2019, 13, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; El-Aziz, S.A.; Elbadry, H.M.; El-Aassar, S.A.; Tamer, T.M. Prevalence, antimicrobial resistance profile, and characterization of multi-drug resistant bacteria from various infected wounds in North Egypt. Saudi J. Biol. Sci. 2022, 29, 2978–2988. [Google Scholar] [CrossRef]
- Sieval, A.; Thanou, M.; Kotze, A.; Verhoef, J.; Brussee, J.; Junginger, H. Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohydr. Polym. 1998, 36, 157–165. [Google Scholar] [CrossRef]
- Hassan, M.A.; Bakhiet, E.K.; Ali, S.G.; Hussien, H.R. Production and characterization of polyhydroxybutyrate (PHB) produced by Bacillus sp. isolated from Egypt. J. Appl. Pharm. Sci. 2016, 5, 46–51. [Google Scholar] [CrossRef]
- Castro, M.P.; Palavecino, N.Z.; Herman, C.; Garro, O.A.; Campos, C.A. Lactic acid bacteria isolated from artisanal dry sausages: Characterization of antibacterial compounds and study of the factors affecting bacteriocin production. Meat Sci. 2011, 87, 321–329. [Google Scholar] [CrossRef]
- Skyttä, E.; Mattila, S.T. A quantitative method for assessing bacteriocins and other food antimicrobials by automated turbidometry. J. Microbiol. Methods 1991, 14, 77–88. [Google Scholar] [CrossRef]
- Rufián, H.J.A.; Morales, F.J. Microtiter plate-based assay for screening antimicrobial activity of melanoidins against E. coli and S. aureus. Food Chem. 2008, 111, 1069–1074. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Soliman, E.A.; Hashem, A.I.; Tamer, T.M. Antimicrobial activity of novel aminated chitosan derivatives for biomedical applications. Adv. Polym. Technol. 2012, 31, 414–428. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]









| Sample Name | N% | C% | H% | C/N% | C/H% |
|---|---|---|---|---|---|
| Ch | 7.17 | 39.05 | 7.21 | 5.45 | 5.42 |
| Mono-NHD-Ch | 2.37 | 20.41 | 6.64 | 8.63 | 3.07 |
| Di-NHD-Ch | 2.09 | 21.89 | 5.20 | 10.46 | 4.21 |
| Inhibition Zone (mm) in Diameter | |||
|---|---|---|---|
| Ch | Mono-NHD-Ch | Di-NHD-Ch | |
| E. coli | 15.8 ± 0.79 | 27.3 ± 1.4 | 29.1 ± 1.5 |
| P. aeruginosa | 13.5 ± 0.68 | 15.3 ± 0.77 | 21.1 ± 1.05 |
| S. aureus | 15.7 ± 0.78 | 19.8 ± 0.98 | 24.6 ± 1.23 |
| B. cereus | 16.4 ± 0.82 | 18.0 ± 0.9 | 23.3 ± 1.65 |
| Sample Concentration (µg/mL) | Inhibition (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | B. cereus | |||||||||
| Ch | Mono-NHD-Ch | Di-NHD-Ch | Ch | Mono-NHD-Ch | Di-NHD-Ch | Ch | Mono-NHD-Ch | Di-NHD-Ch | Ch | Mono-NHD-Ch | Di-NHD-Ch | |
| 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 50 | 18.23 ± 0.91 | 41.37 ± 2.07 | 48.18 ± 2.41 | 24.12 ± 1.21 | 27.1 ± 1.36 | 39.23 ± 1.96 | 0 | 16.37 ± 0.82 | 24.37 ± 1.22 | 0 | 0 | 0 |
| 100 | 28.78 ± 1.44 | 68.13 ± 3.41 | 71.23 ± 3.56 | 33.7 ± 1.69 | 35.31 ± 1.77 | 41.22 ± 2.06 | 18.37 ± 0.92 | 33.47 ± 1.67 | 39.71 ± 1.99 | 37.21 ± 1.86 | 39.27 ± 1.96 | 43.54 ± 2.18 |
| 150 | 37.12 ± 1.86 | 71.47 ± 3.57 | 79.38 ± 3.97 | 35.69 ± 1.78 | 40.4 ± 2.02 | 55.71 ± 2.79 | 32.76 ± 1.64 | 41.38 ± 2.07 | 51.37 ± 2.57 | 52.31 ± 2.62 | 57.31 ± 2.87 | 74.27 ± 3.71 |
| 200 | 56.27± 2.81 | 79.21 ± 3.96 | 92.37 ± 4.62 | 40.01 ± 2.0 | 52.34 ± 2.62 | 78.16 ± 3.91 | 49.38 ± 2.47 | 49.31 ± 1.47 | 72.39 ± 3.62 | 58.23 ± 2.91 | 61.35 ± 3.07 | 91.32 ± 4.57 |
| 250 | 61.37 ± 3.07 | 82.52 ± 4.13 | 98.92 ± 4.95 | 49.67 ± 2.48 | 61.32 ± 3.07 | 90.06 ± 4.50 | 56.27 ± 2.81 | 63.87 ± 3.19 | 99.7 ± 4.99 | 61.35 ± 3.07 | 72.31 ± 3.62 | 97.45 ± 4.87 |
| Weight (mg) | Ch | Mono-NHD-Ch | Di-NHD-Ch | |||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | |
| 1 | 99.473 | 3.389 | 94.051 | 1.130 | 49.473 | 3.087 |
| 0.5 | 99.783 | 0.217 | 98.270 | 0.380 | 73.159 | 4.795 |
| 0.25 | 100.615 | 0.615 | 99.832 | 0.169 | 81.926 | 2.295 |
| 0.125 | 100.936 | 0.924 | 101.930 | 0.600 | 92.642 | 2.982 |
| 0.0625 | 101.695 | 0.605 | 102.500 | 0.490 | 99.096 | 0.534 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, H.; El-Sigeny, S.; Shoman, S.; Abu-Serie, M.M.; Tamer, T.M. Preparation, Characterization, and Bio Evaluation of Fatty N- Hexadecanyl Chitosan Derivatives for Biomedical Applications. Polymers 2022, 14, 4011. https://doi.org/10.3390/polym14194011
Mansour H, El-Sigeny S, Shoman S, Abu-Serie MM, Tamer TM. Preparation, Characterization, and Bio Evaluation of Fatty N- Hexadecanyl Chitosan Derivatives for Biomedical Applications. Polymers. 2022; 14(19):4011. https://doi.org/10.3390/polym14194011
Chicago/Turabian StyleMansour, Hanaa, Samia El-Sigeny, Sarah Shoman, Marwa M. Abu-Serie, and Tamer M. Tamer. 2022. "Preparation, Characterization, and Bio Evaluation of Fatty N- Hexadecanyl Chitosan Derivatives for Biomedical Applications" Polymers 14, no. 19: 4011. https://doi.org/10.3390/polym14194011
APA StyleMansour, H., El-Sigeny, S., Shoman, S., Abu-Serie, M. M., & Tamer, T. M. (2022). Preparation, Characterization, and Bio Evaluation of Fatty N- Hexadecanyl Chitosan Derivatives for Biomedical Applications. Polymers, 14(19), 4011. https://doi.org/10.3390/polym14194011