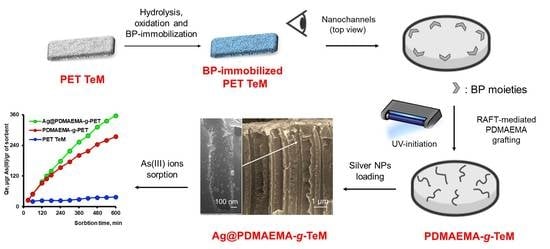
Hybrid PET Track-Etched Membranes Grafted by Well-Defined Poly(2-(dimethylamino)ethyl methacrylate) Brushes and Loaded with Silver Nanoparticles for the Removal of As(III)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Irradiation and Track-Etching of PET Films
2.3. Grafting of PDMAEMA from the Nanochannels of PET TeMs (PDMAEMA-g-TeMs)
2.4. Quaternization of PDMAEMA-g-TeMs (Q-PDMAEMA-g-TeMs)
2.5. Loading of Ag NPs onto PDMAEMA-g-TeMs (Ag@PDMAEMA-g-TeMs)
2.6. Batch Absorption Experiments
2.7. Characterizations
3. Results
3.1. Characterization of the Composite Membranes
3.2. Kinetic Study of As(III) Removal
3.3. Equilibrium Studies of As(III) Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TeM | track-etched membrane |
| PET | Poly(ethylene terephthalate) |
| DMAEMA | 2-(dimethyamino)ethyl methacrylate |
| PDMAEMA | poly-2-(dimethyamino)ethyl methacrylate |
| PDMAEMA-g-PET | poly(2-(dimethyamino)ethyl methacrylate) grafted PETtrack-etched membranes |
| Ag@PDMAEMA-g-PET | poly(2-(dimethyamino)ethyl methacrylate) grafted track-etched membranes loaded with silver nanoparticles |
| RA1 | O-ethyl-S-(1-methoxycarbonyl) ethyl dithiocarbonate |
| TGA | thermogravimetric analysis |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| SEM | scanning electron microscopy |
| EDX | energy dispersive X-ray analysis |
| RDRP | reversible-deactivation radical polymerization |
| RAFT | reversible addition fragmentation chain transfer polymerization |
| CTAs | chain transfer agents |
| BP | benzophenone |
| pHPZC | pH of zero point |
| NPs | nanoparticles |
| Qe | amount of As(III) adsorbed by the unit mass of copper (mg/g) |
| C0 | feed As(III) concentration (mg/L) |
| Ce | concentration of As(III) in aliquots (mg/L) |
| DC | degree of crystallinity (%) |
| L | average crystallite size (nm) |
| adsorption capacity at time (mg/g) | |
| First-order reaction rate constant (min−1) | |
| pseudo-second-order rate constant of adsorption () | |
| initial rate of the adsorption process, mg/g × min | |
| desorption constant (g·mmol−1) | |
| Ra | roughness of the composite (nm) |
| b | constant related to the energy of adsorption (i.e., Langmuir constant (L/µg)) |
| Ce | equilibrium concentration of adsorbate (mg/L) |
| maximum monolayer coverage capacity (mg/g) | |
| Freundlich isotherm constant related to the adsorption capacity (µg/g) | |
| adsorption capacity of the Dubinin–Radushkevich monolayer (µg/g) | |
| constant associated with the free energy of sorption (mol2/kJ2) | |
| free energy of adsorption (kJ/mol) |
References
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- McLellan, F. Arsenic contamination affects millions in Bangladesh. Lancet 2002, 359, 1127. [Google Scholar] [CrossRef]
- Siddique, T.; Dutta, N.K.; Roy Choudhury, N. Nanofiltration for Arsenic Removal: Challenges, Recent Developments, and Perspectives. Nanomaterials 2020, 10, 1323. [Google Scholar] [CrossRef] [PubMed]
- Tanne, N.; Xu, R.; Zhou, M.; Zhang, P.; Wang, X.; Wen, X. Influence of pore size and membrane surface properties on arsenic removal by nanofiltration membranes. Front. Environ. Sci. Eng. 2019, 13, 19. [Google Scholar] [CrossRef]
- Ma, T.; Janot, J.; Balme, S. Track-Etched Nanopore/Membrane: From Fundamental to Applications. Small Methods 2020, 4, 2000366. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Barsbay, M.; Aimanova, N.A.; Zdorovets, M.V. Application of Silver-Loaded Composite Track-Etched Membranes for Photocatalytic Decomposition of Methylene Blue under Visible Light. Membranes 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Altynbaeva, L.S.; Barsbay, M.; Aimanova, N.A.; Jakupova, Z.Y.; Nurpeisova, D.T.; Zdorovets, M.V.; Mashentseva, A.A. A Novel Cu2O/ZnO@PET Composite Membrane for the Photocatalytic Degradation of Carbendazim. Nanomaterials 2022, 12, 1724. [Google Scholar] [CrossRef] [PubMed]
- Barsbay, M.; Güven, O. Grafting in confined spaces: Functionalization of nanochannels of track-etched membranes. Radiat. Phys. Chem. 2014, 105, 26–30. [Google Scholar] [CrossRef]
- Feng, C.; Li, Y.; Yang, D.; Hu, J.; Zhang, X.; Huang, X. Well-defined graft copolymers: From controlled synthesis to multipurpose applications. Chem. Soc. Rev. 2011, 40, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Barsbay, M.; Güven, O. Nanostructuring of polymers by controlling of ionizing radiation-induced free radical polymerization, copolymerization, grafting and crosslinking by RAFT mechanism. Radiat. Phys. Chem. 2020, 169, 107816. [Google Scholar] [CrossRef]
- Kavaklı, C.; Barsbay, M.; Tilki, S.; Güven, O.; Kavaklı, P.A. Activation of Polyethylene/Polypropylene Nonwoven Fabric by Radiation-Induced Grafting for the Removal of Cr(VI) from Aqueous Solutions. Water Air Soil Pollut. 2016, 227, 473. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O. Modification of polystyrene cell-culture-dish surfaces by consecutive grafting of poly(acrylamide)/poly(N-isopropylacrylamide) via reversible addition-fragmentation chain transfer-mediated polymerization. Eur. Polym. J. 2021, 147, 110330. [Google Scholar] [CrossRef]
- Söylemez, M.A.; Barsbay, M.; Güven, O. Preparation of well-defined erythromycin imprinted non-woven fabrics via radiation-induced RAFT-mediated grafting. Radiat. Phys. Chem. 2018, 142, 77–81. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O.; Bessbousse, H.; Wade, T.L.; Beuneu, F.; Clochard, M.-C. Nanopore size tuning of polymeric membranes using the RAFT-mediated radical polymerization. J. Memb. Sci. 2013, 445, 135–145. [Google Scholar] [CrossRef]
- Alem, H.; Duwez, A.-S.; Lussis, P.; Lipnik, P.; Jonas, A.M.; Demoustier-Champagne, S. Microstructure and thermo-responsive behavior of poly(N-isopropylacrylamide) brushes grafted in nanopores of track-etched membranes. J. Memb. Sci. 2008, 308, 75–86. [Google Scholar] [CrossRef]
- Yang, Q.; Ulbricht, M. Novel Membrane Adsorbers with Grafted Zwitterionic Polymers Synthesized by Surface-Initiated ATRP and Their Salt-Modulated Permeability and Protein Binding Properties. Chem. Mater. 2012, 24, 2943–2951. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Manan, F.A. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Buha Djordjevic, A.; Crisponi, G.; Alexander, J.; Bjørklund, G.; Aaseth, J. Arsenic Toxicity: Molecular Targets and Therapeutic Agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Li, X.-F.; Cullen, W.R.; Weinfeld, M.; Le, X.C. Arsenic Binding to Proteins. Chem. Rev. 2013, 113, 7769–7792. [Google Scholar] [CrossRef]
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chiu, V.Q.; Hering, J.G. Arsenic Adsorption and Oxidation at Manganite Surfaces. 1. Method for Simultaneous Determination of Adsorbed and Dissolved Arsenic Species. Environ. Sci. Technol. 2000, 34, 2029–2034. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, M.; Liu, R.; Wang, D.; Lin, X.; Liu, W.; Ma, L.; Li, Y.; Huang, Y. Modified native cellulose fibers—A novel efficient adsorbent for both fluoride and arsenic. J. Hazard. Mater. 2011, 185, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Wu, X.; Wei, J.; Zhang, H.; Cui, L. Preparation and characterization of hydrophilicity fibers based on 2-(dimethyamino)ethyl mathacrylate grafted polypropylene by UV- irradiation for removal of Cr(VI) and as(V). J. Polym. Res. 2016, 23, 199. [Google Scholar] [CrossRef]
- Habuda-Stanić, M.; Nujić, M. Arsenic removal by nanoparticles: A review. Environ. Sci. Pollut. Res. 2015, 22, 8094–8123. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.E.; Korfiatis, G.P.; Patel, M.; Lippincott, L.; Meng, X. Adsorption of As(V) and As(III) by nanocrystalline titanium dioxide. Water Res. 2005, 39, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, Q.; Gao, S.; Shang, J.K. As(III) removal by hydrous titanium dioxide prepared from one-step hydrolysis of aqueous TiCl4 solution. Water Res. 2010, 44, 5713–5721. [Google Scholar] [CrossRef] [PubMed]
- Jegadeesan, G.; Al-Abed, S.R.; Sundaram, V.; Choi, H.; Scheckel, K.G.; Dionysiou, D.D. Arsenic sorption on TiO2 nanoparticles: Size and crystallinity effects. Water Res. 2010, 44, 965–973. [Google Scholar] [CrossRef]
- Prasad, B.; Ghosh, C.; Chakraborty, A.; Bandyopadhyay, N.; Ray, R.K. Adsorption of arsenite (As3+) on nano-sized Fe2O3 waste powder from the steel industry. Desalination 2011, 274, 105–112. [Google Scholar] [CrossRef]
- Shipley, H.J.; Yean, S.; Kan, A.T.; Tomson, M.B. Adsorption of arsenic to magnetite nanoparticles: Effect of particle concentration, ph, ionic strength, and temperature. Environ. Toxicol. Chem. 2009, 28, 509. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.R.; Yanful, E.K. Arsenic removal from aqueous solutions by adsorption on magnetite nanoparticles. Water Environ. J. 2011, 25, 429–437. [Google Scholar] [CrossRef]
- Sun, W.; Li, Q.; Gao, S.; Shang, J.K. Exceptional arsenic adsorption performance of hydrous cerium oxide nanoparticles: Part B. Integration with silica monoliths and dynamic treatment. Chem. Eng. J. 2012, 185–186, 136–143. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Wang, L.; Sheng, G.; Liu, J.; Yu, H.; Huang, X.-J. Enhanced arsenic removal from water by hierarchically porous CeO2–ZrO2 nanospheres: Role of surface- and structure-dependent properties. J. Hazard. Mater. 2013, 260, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Martinson, C.A.; Reddy, K.J. Adsorption of arsenic(III) and arsenic(V) by cupric oxide nanoparticles. J. Colloid Interface Sci. 2009, 336, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Mashentseva, A.A.; Barsbay, M.; Zdorovets, M.V.; Zheltov, D.A.; Güven, O. Cu/CuO Composite Track-Etched Membranes for Catalytic Decomposition of Nitrophenols and Removal of As(III). Nanomaterials 2020, 10, 1552. [Google Scholar] [CrossRef] [PubMed]
- Russakova, A.V.; Altynbaeva, L.S.; Barsbay, M.; Zheltov, D.A.; Zdorovets, M.V.; Mashentseva, A.A. Kinetic and isotherm study of as(Iii) removal from aqueous solution by pet track-etched membranes loaded with copper microtubes. Membranes 2021, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Dutta, A.; Bhaumik, A. Self-assembled mesoporous γ-Al2O3 spherical nanoparticles and their efficiency for the removal of arsenic from water. J. Hazard. Mater. 2012, 201–202, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, Q.; Gao, S.; Shang, J.K. Strong adsorption of arsenic species by amorphous zirconium oxide nanoparticles. J. Ind. Eng. Chem. 2012, 18, 1418–1427. [Google Scholar] [CrossRef]
- Olyaie, E.; Banejad, H.; Afkhami, A.; Rahmani, A.; Khodaveisi, J. Development of a cost-effective technique to remove the arsenic contamination from aqueous solutions by calcium peroxide nanoparticles. Sep. Purif. Technol. 2012, 95, 10–15. [Google Scholar] [CrossRef]
- Tandon, P.K.; Shukla, R.C.; Singh, S.B. Removal of Arsenic(III) from Water with Clay-Supported Zerovalent Iron Nanoparticles Synthesized with the Help of Tea Liquor. Ind. Eng. Chem. Res. 2013, 52, 10052–10058. [Google Scholar] [CrossRef]
- Arsiya, F.; Hossein, M.; Sobhani, S. Arsenic (III) Adsorption Using Palladium Nanoparticles from Aqueous Solution. J. Water Environ. Nanotechnol. 2017, 2, 166–173. [Google Scholar]
- Mukherjee, T.; Ghosh, G.; Mukherjee, R.; Das, T.K. Study of arsenic (III) removal by monolayer protected silver nanoadsorbent and its execution on prokaryotic system. J. Environ. Manage. 2019, 244, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, R.; Arul Jothi, N.; Jayavignesh, V.; Karthikaiselvi, K.; Antony, G.I.; Sharmila, P.R.; Kavitha, S.; Swaminathan, K. As(V) removal using carbonized yeast cells containing silver nanoparticles. Water Res. 2011, 45, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Attatsi, I.K.; Nsiah, F. Application of silver nanoparticles toward Co(II) and Pb(II) ions contaminant removal in groundwater. Appl. Water Sci. 2020, 10, 152. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Sundaram, S.; Yadav, K.K.; Srivastav, A.L. An overview of silver nano-particles as promising materials for water disinfection. Environ. Technol. Innov. 2021, 23, 101721. [Google Scholar] [CrossRef]
- Barsbay, M.; Çaylan Özgür, T.; Sütekin, S.D.; Güven, O. Effect of brush length of stabilizing grafted matrix on size and catalytic activity of metal nanoparticles. Eur. Polym. J. 2020, 134, 109811. [Google Scholar] [CrossRef]
- Gao, J.; Huddleston, N.E.; White, E.M.; Pant, J.; Handa, H.; Locklin, J. Surface Grafted Antimicrobial Polymer Networks with High Abrasion Resistance. ACS Biomater. Sci. Eng. 2016, 2, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Pahnke, J.; Rühe, J. Attachment of Polymer Films to Aluminium Surfaces by Photochemically Active Monolayers of Phosphonic Acids. Macromol. Rapid Commun. 2004, 25, 1396–1401. [Google Scholar] [CrossRef]
- Prucker, O.; Naumann, C.A.; Rühe, J.; Knoll, W.; Frank, C.W. Photochemical attachment of polymer films to solid surfaces via monolayers of benzophenone derivatives. J. Am. Chem. Soc. 1999, 121, 8766–8770. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Yeszhanov, A.B.; Zdorovets, M.V.; Gorin, Y.G.; Güven, O.; Dosmagambetova, S.S.; Khlebnikov, N.A.; Serkov, K.V.; Krasnopyorova, M.V.; Milts, O.S.; et al. Modification of PET ion track membranes for membrane distillation of low-level liquid radioactive wastes and salt solutions. Sep. Purif. Technol. 2019, 227, 115694. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Mashentseva, A.A.; Güven, O.; Taltenov, A.A. UV-induced graft polymerization of acrylic acid in the sub-micronchannels of oxidized PET track-etched membrane. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 365, 419–423. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Mashentseva, A.A.; Güven, O.; Zdorovets, M.V.; Taltenov, A.A. Enhancing hydrophilicity and water permeability of PET track-etched membranes by advanced oxidation process. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 365, 651–655. [Google Scholar] [CrossRef]
- Mashentseva, A.A. Effect of the Oxidative Modification and Activation of Templates Based on Poly (ethylene terephthalate) Track-Etched Membranes on the Electroless Deposition of Copper and the Catalytic Properties of Composite Membranes. Pet. Chem. 2019, 59, 1337–1344. [Google Scholar] [CrossRef]
- Kavaklı, C.; Akkaş Kavaklı, P.; Turan, B.D.; Hamurcu, A.; Güven, O. Quaternized dimethylaminoethyl methacrylate strong base anion exchange fibers for As(V) adsorption. Radiat. Phys. Chem. 2014, 102, 84–95. [Google Scholar] [CrossRef]
- Agrawal, S.; Singh, N.B. Removal of arsenic from aqueous solution by an adsorbent nickel ferrite-polyaniline nanocomposite. Indian J. Chem. Technol. 2016, 23, 374–383. [Google Scholar]
- Mashentseva, A.A.; Shlimas, D.I.; Kozlovskiy, A.L.; Zdorovets, M.V.; Russakova, A.V.; Kassymzhanov, M.; Borisenko, A.N. Electron Beam Induced Enhancement of the Catalytic Properties of Ion-Track Membranes Supported Copper Nanotubes in the Reaction of the P-Nitrophenol Reduction. Catalysts 2019, 9, 737. [Google Scholar] [CrossRef]
- Muhammed Shafi, P.; Chandra Bose, A. Impact of crystalline defects and size on X-ray line broadening: A phenomenological approach for tetragonal SnO2 nanocrystals. AIP Adv. 2015, 5, 057137. [Google Scholar] [CrossRef]
- Stenzel, M.H. RAFT polymerization: An avenue to functional polymeric micelles for drug delivery. Chem. Commun. 2008, 3486–3503. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Su, L.; Luo, Y.; Cao, X.; Hu, S.; Li, S.; Liang, Y.; Liu, S.; Xu, W.; Qin, D.; et al. Biologically Mediated RAFT Polymerization for Electrochemical Sensing of Kinase Activity. Anal. Chem. 2022, 94, 6200–6205. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Luo, Y.; Cao, X.; Chen, Z.; Huang, Y.; Niu, L. Bioinspired Electro-RAFT Polymerization for Electrochemical Sensing of Nucleic Acids. ACS Appl. Mater. Interfaces 2021, 13, 54794–54800. [Google Scholar] [CrossRef]
- Söylemez, M.A.; Güven, O.; Barsbay, M. Method for preparing a well-defined molecularly imprinted polymeric system via radiation-induced RAFT polymerization. Eur. Polym. J. 2018, 103, 21–30. [Google Scholar] [CrossRef]
- Söylemez, M.A.; Okan, M.; Güven, O.; Barsbay, M. Synthesis of well-defined molecularly imprinted bulk polymers for the removal of azo dyes from water resources. Curr. Res. Green Sustain. Chem. 2021, 4, 100196. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Advanced Materials by Atom Transfer Radical Polymerization. Adv. Mater. 2018, 30, 1706441. [Google Scholar] [CrossRef] [PubMed]
- Destarac, M.; Matioszek, D.; Vila, X.; Ruchmann-Sternchuss, J.; Zard, S.Z. How Can Xanthates Control the RAFT Polymerization of Methacrylates? In Reversible Deactivation Radical Polymerization: Mechanisms and Synthetic Methodologies; Matyjaszewski, K., Gao, H., Sumerlin, B.S., Tsarevsky, N.V., Eds.; Oxford University Press Inc.: New York, NY, USA, 2018; pp. 291–305. [Google Scholar]
- Kuşçuoğlu, C.K.; Güner, H.; Söylemez, M.A.; Güven, O.; Barsbay, M. A smartphone-based colorimetric PET sensor platform with molecular recognition via thermally initiated RAFT-mediated graft copolymerization. Sens. Actuators B Chem. 2019, 296, 126653. [Google Scholar] [CrossRef]
- Schneider, M.H.; Tran, Y.; Tabeling, P. Benzophenone Absorption and Diffusion in Poly(dimethylsiloxane) and Its Role in Graft Photo-polymerization for Surface Modification. Langmuir 2011, 27, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.H.; Liu, N.; Sun, G. UV-induced graft polymerization of acrylamide on cellulose by using immobilized benzophenone as a photo-initiator. Eur. Polym. J. 2009, 45, 2443–2449. [Google Scholar] [CrossRef]
- Kim, J.; Hanna, J.A.; Byun, M.; Santangelo, C.D.; Hayward, R.C. Designing Responsive Buckled Surfaces by Halftone Gel Lithography. Science 2012, 335, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Brandstetter, T.; Böhmer, S.; Prucker, O.; Bissé, E.; zur Hausen, A.; Alt-Mörbe, J.; Rühe, J. A polymer-based DNA biochip platform for human papilloma virus genotyping. J. Virol. Methods 2010, 163, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Virkar, A.; Ling, M.-M.; Locklin, J.; Bao, Z. Oligothiophene based organic semiconductors with cross-linkable benzophenone moieties. Synth. Met. 2008, 158, 958–963. [Google Scholar] [CrossRef]
- Kodama, Y.; Barsbay, M.; Güven, O. Poly(2-hydroxyethyl methacrylate) (PHEMA) grafted polyethylene/polypropylene (PE/PP) nonwoven fabric by γ-initiation: Synthesis, characterization and benefits of RAFT mediation. Radiat. Phys. Chem. 2014, 105, 31–38. [Google Scholar] [CrossRef]
- Stawski, D.; Nowak, A. Thermal properties of poly(N,N-dimethylaminoethyl methacrylate). PLoS ONE 2019, 14, e0217441. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.; Shi, M.; Xu, X.; He, S.; Yang, C.; Wang, L.; Wu, R.; Wang, W.; Wang, J. DMAEMA-grafted cellulose as an imprinted adsorbent for the selective adsorption of 4-nitrophenol. Cellulose 2021, 28, 6481–6498. [Google Scholar] [CrossRef]
- Xing, L.; Wang, Y.; Wang, S.; Zhang, Y.; Mao, S.; Wang, G.; Liu, J.; Huang, L.; Li, H.; Belfiore, L.A.; et al. Effects of Modified Graphene Oxide on Thermal and Crystallization Properties of PET. Polymers 2018, 10, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, P.; Li, G.; Lv, L.; Ji, H.; Li, Z.; Chen, S.; Chu, S. Effect of DMAEMA content and polymerization mode on morphologies and properties of pH and temperature double-sensitive cellulose-based hydrogels. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 57, 207–216. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Mashentseva, A.A.; Güven, O.; Niyazova, D.T.; Barsbay, M.; Zdorovets, M.V. The effect of oxidizing agents/systems on the properties of track-etched PET membranes. Polym. Degrad. Stab. 2014, 107, 150–157. [Google Scholar] [CrossRef]
- Ryan, B.J.; Poduska, K.M. Roughness effects on contact angle measurements. Am. J. Phys. 2008, 76, 1074–1077. [Google Scholar] [CrossRef]
- Tu, Q.; Wang, J.-C.; Liu, R.; He, J.; Zhang, Y.; Shen, S.; Xu, J.; Liu, J.; Yuan, M.-S.; Wang, J. Antifouling properties of poly(dimethylsiloxane) surfaces modified with quaternized poly(dimethylaminoethyl methacrylate). Colloids Surf. B Biointerfaces 2013, 102, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Ayres, N.; Boyes, S.G.; Brittain, W.J. Stimuli-Responsive Polyelectrolyte Polymer Brushes Prepared via Atom-Transfer Radical Polymerization. Langmuir 2007, 23, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Gadre, K.S.; Alford, T.L. Contact angle measurements for adhesion energy evaluation of silver and copper films on parylene- n and SiO2 substrates. J. Appl. Phys. 2003, 93, 919–923. [Google Scholar] [CrossRef]
- Plamper, F.A.; Schmalz, A.; Penott-Chang, E.; Drechsler, M.; Jusufi, A.; Ballauff, M.; Müller, A.H.E. Synthesis and Characterization of Star-Shaped Poly(N,N-dimethylaminoethyl methacrylate) and Its Quaternized Ammonium Salts. Macromolecules 2007, 40, 5689–5697. [Google Scholar] [CrossRef]
- Koufakis, E.; Manouras, T.; Anastasiadis, S.H.; Vamvakaki, M. Film Properties and Antimicrobial Efficacy of Quaternized PDMAEMA Brushes: Short vs Long Alkyl Chain Length. Langmuir 2020, 36, 3482–3493. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-F.; Hu, H.-Q.; Li, Y.; Wan, L.-S.; Xu, Z.-K. Membrane surface with antibacterial property by grafting polycation. J. Memb. Sci. 2011, 376, 132–141. [Google Scholar] [CrossRef]
- Khan, T.A.; Chaudhry, S.A.; Ali, I. Equilibrium uptake, isotherm and kinetic studies of Cd(II) adsorption onto iron oxide activated red mud from aqueous solution. J. Mol. Liq. 2015, 202, 165–175. [Google Scholar] [CrossRef]
- Leiva, E.; Tapia, C.; Rodríguez, C. Highly Efficient Removal of Cu(II) Ions from Acidic Aqueous Solution Using ZnO Nanoparticles as Nano-Adsorbents. Water 2021, 13, 2960. [Google Scholar] [CrossRef]
- Panagiotaras, D.; Panagopoulos, G.; Papoulis, D.; Avramidis, P. Arsenic Geochemistry in Groundwater System. In Geochemistry—Earth’s System Processes; Panagiotaras, D., Ed.; InTech: London, UK, 2012; pp. 27–38. [Google Scholar]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab. J. Chem. 2017, 10, S3381–S3393. [Google Scholar] [CrossRef]
- Soni, R.; Shukla, D.P. Data on Arsenic(III) removal using zeolite-reduced graphene oxide composite. Data Br. 2019, 22, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Pillewan, P.; Mukherjee, S.; Roychowdhury, T.; Das, S.; Bansiwal, A.; Rayalu, S. Removal of As(III) and As(V) from water by copper oxide incorporated mesoporous alumina. J. Hazard. Mater. 2011, 186, 367–375. [Google Scholar] [CrossRef]
- Podder, M.S.; Majumder, C.B. Biosorption of As(III) and As(V) on the surface of TW/MnFe2O4 composite from wastewater: Kinetics, mechanistic and thermodynamics. Appl. Water Sci. 2017, 7, 2689–2715. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, J.; Li, M. Fundamental Studies of Novel Zwitterionic Hybrid Membranes: Kinetic Model and Mechanism Insights into Strontium Removal. Sci. World J. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, X.-B.; Ding, L.; Luo, S.-L. Application of Nanotechnology in the Removal of Heavy Metal from Water. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–147. [Google Scholar]
- Mashentseva, A.A.; Aimanova, N.A.; Parmanbek, N.; Temirgaziyev, B.S.; Barsbay, M.; Zdorovets, M.V. Serratula coronata L. Mediated Synthesis of ZnO Nanoparticles and Their Application for the Removal of Alizarin Yellow R by Photocatalytic Degradation and Adsorption. Nanomaterials 2022, 12, 3293. [Google Scholar] [CrossRef]
- Villabona-Ortíz, Á.; Figueroa-Lopez, K.J.; Ortega-Toro, R. Kinetics and Adsorption Equilibrium in the Removal of Azo-Anionic Dyes by Modified Cellulose. Sustainability 2022, 14, 3640. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The sorption of lead(II) ions on peat. Water Res. 1999, 33, 578–584. [Google Scholar] [CrossRef]
- Miller, S.M.; Zimmerman, J.B. Novel, bio-based, photoactive arsenic sorbent: TiO2-impregnated chitosan bead. Water Res. 2010, 44, 5722–5729. [Google Scholar] [CrossRef] [PubMed]
- Savina, I.N.; English, C.J.; Whitby, R.L.D.; Zheng, Y.; Leistner, A.; Mikhalovsky, S.V.; Cundy, A.B. High efficiency removal of dissolved As(III) using iron nanoparticle-embedded macroporous polymer composites. J. Hazard. Mater. 2011, 192, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Malana, M.A.; Qureshi, R.B.; Ashiq, M.N. Adsorption studies of arsenic on nano aluminium doped manganese copper ferrite polymer (MA, VA, AA) composite: Kinetics and mechanism. Chem. Eng. J. 2011, 172, 721–727. [Google Scholar] [CrossRef]










| Kinetic Model | Linearized Equation | Model Parameters | Value | |
|---|---|---|---|---|
| Ag@DMAE- MA-g-PET | DMAEMA-g-PET | |||
| Pseudo-first-order | , min−1 | 0.007 | 0.005 | |
| , mg/g | 762.7 | 349.6 | ||
| R2 | 0.74 | 0.94 | ||
| Pseudo-second-order | , g/mg × min | 1.50 | 2.82 | |
| , mg/g | 769.2 | 555.6 | ||
| R2 | 0.99 | 0.99 | ||
| Intra-particle diffusion | + C | , mg/(g × h0.5) | 16.29 | 13.38 |
| , mg/g | 75.12 | 53.3 | ||
| R2 | 0.99 | 0.99 | ||
| Isotherm Model | Linearized Equation | Model Parameters | Value | |
|---|---|---|---|---|
| Ag@ DMAEMA-g-PET | DMAEMA -g-PET | |||
| Langmuir | , µg/g | 79.37 | 39.84 | |
| b, L/mg | 77.85 | 10.02 | ||
| R2 | 0.89 | 0.92 | ||
| Freundlich | , µg/g | 18.46 | 2.84 | |
| 0.64 | 1.00 | |||
| R2 | 0.99 | 1.0 | ||
| Dubinin–Radushkevich | Qd, µg/g | 107.39 | 107.28 | |
| , mol2/kJ2 | 0.002 | 0.010 | ||
| , kJ/mol | 14.74 | 6.93 | ||
| R2 | 0.79 | 0.93 | ||
| Composite Adsorbent | Sorption Conditions | Qe, mg/g | Ref. | |
|---|---|---|---|---|
| Initial Concentration of Adsorbate, ppm | Amount of Adsorbent Utilized, mg | |||
| TiO2-impregnated chitosan bead | 100.0 | 25.0 | 2.1 | [96] |
| α-Fe2O3–polymer monolith | 4000.0 | 500.0 | 2.7 | [97] |
| Aluminum doped manganese copper ferrite/polymer composite | 0.2 | 50.0 | 0.053 | [98] |
| Cu/PET TeM | 50.0 | 3.8 | 0.52 | [35] |
| Cu/Ox_PET TeM | 4.0 | 0.80 | ||
| Ag@PDMAEMA-g-PET | 50.0 | 9.0 | 0.357 | This study |
| PDMAEMA-g-PET | 50.0 | 6.4 | 0.274 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parmanbek, N.; Sütekin, D.S.; Barsbay, M.; Mashentseva, A.A.; Zheltov, D.A.; Aimanova, N.A.; Jakupova, Z.Y.; Zdorovets, M.V. Hybrid PET Track-Etched Membranes Grafted by Well-Defined Poly(2-(dimethylamino)ethyl methacrylate) Brushes and Loaded with Silver Nanoparticles for the Removal of As(III). Polymers 2022, 14, 4026. https://doi.org/10.3390/polym14194026
Parmanbek N, Sütekin DS, Barsbay M, Mashentseva AA, Zheltov DA, Aimanova NA, Jakupova ZY, Zdorovets MV. Hybrid PET Track-Etched Membranes Grafted by Well-Defined Poly(2-(dimethylamino)ethyl methacrylate) Brushes and Loaded with Silver Nanoparticles for the Removal of As(III). Polymers. 2022; 14(19):4026. https://doi.org/10.3390/polym14194026
Chicago/Turabian StyleParmanbek, Nursanat, Duygu S. Sütekin, Murat Barsbay, Anastassiya A. Mashentseva, Dmitriy A. Zheltov, Nurgulim A. Aimanova, Zhanar Ye. Jakupova, and Maxim V. Zdorovets. 2022. "Hybrid PET Track-Etched Membranes Grafted by Well-Defined Poly(2-(dimethylamino)ethyl methacrylate) Brushes and Loaded with Silver Nanoparticles for the Removal of As(III)" Polymers 14, no. 19: 4026. https://doi.org/10.3390/polym14194026
APA StyleParmanbek, N., Sütekin, D. S., Barsbay, M., Mashentseva, A. A., Zheltov, D. A., Aimanova, N. A., Jakupova, Z. Y., & Zdorovets, M. V. (2022). Hybrid PET Track-Etched Membranes Grafted by Well-Defined Poly(2-(dimethylamino)ethyl methacrylate) Brushes and Loaded with Silver Nanoparticles for the Removal of As(III). Polymers, 14(19), 4026. https://doi.org/10.3390/polym14194026