Study of the Microscopic Mechanism of Natural Rubber (Cis-1, 4-Polyisoprene, NR)/Polyethylene (PE) Modified Asphalt from the Perspective of Simulation
Abstract
:1. Introduction
2. Simulation
2.1. Model of Asphalt
2.2. Models of Rubber
2.3. Models of PE
2.4. Models of the Mixture
- The establishment of initial polymer model.
- Geometric optimization.
- The simulation of annealing.
- In this procedure, the pressure (1.01 × 10−4 Gpa) and temperature of the system remain constant [45].
- NVT+NPT dynamics simulation.
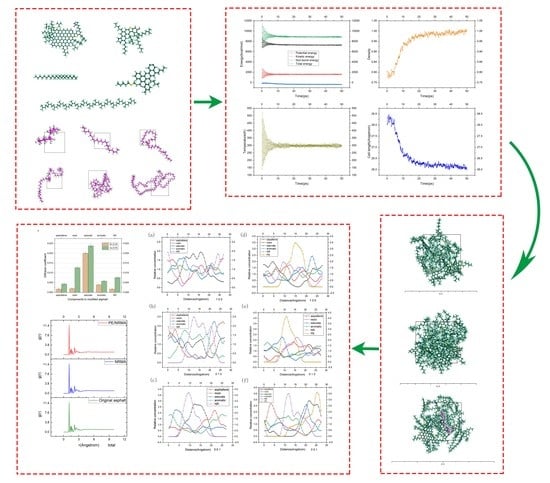
- At ambient temperature and standard atmospheric pressure, 50 ps of MD simulation was performed to bring the model closer to its natural state [46,47]. Taking the original asphalt model as an example, after the above steps were performed, parameters such as the energy, density, and others for the system reached a stable state, as shown in Figure 4. For example, the density of the original asphalt model increased and stabilized at about 1.0, which coincided with the actual situation.
- The calculation and analysis of parameters.
- After the molecular dynamic simulation, parameters related to this study were analyzed and calculated, such as the solubility parameters and radial distribution function, etc., according to the stable model generated at the end of the simulation process.

3. Results and Discussion
3.1. Solubility Parameters
3.2. Radial Distribution Function
3.3. Diffusion Coefficient
3.4. Concentration Distribution
4. Conclusions
- The optimal degree of polymerization of PE in this study model is 12. In this case, the solubility parameter between PE and NR-modified asphalt is the smallest at 0.14 < 1.3~2.1 (J/cm3) 1/2.
- The three models in the paper are typical amorphous substances with structures of short-range order and long-range disorder. According to the RDF diagram, when = 1.11, the functions for the three models all have sharp peaks, which are 11.76, 11.74, and 11.8, respectively.
- In NR-modified asphalt, the diffusion coefficient of saturate is the largest, at far higher than that of other types of molecules, and its value reaches 0.0201. In addition, the molecular velocity of asphaltenes, resins, and rubbers are about the same, showing that their diffusion coefficients are about 0.0018. NR is the slowest molecule.
- In PE/NR-modified asphalt, the movement of molecules changed significantly. The diffusion coefficient of resin molecules increased considerably from 0.0020 to 0.0127. The most noticeable feature is a specific adsorption phenomenon between the PE molecule and light components.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porto, M.; Caputo, P.; Loise, V.; Eskandarsefat, S.; Teltayev, B.; Rossi, C.O. Bitumen and bitumen modification: A review on latest advances. Appl. Sci. 2019, 9, 742. [Google Scholar] [CrossRef]
- Presti, D.L. Recycled tyre rubber modified bitumens for road asphalt mixtures: A literature review. Constr. Build. Mater. 2013, 49, 863–881. [Google Scholar] [CrossRef]
- Jiang, W.; Yuan, D.D.; Shan, J.H.; Ye, W.L.; Lu, H.H.; Sha, A.M. Experimental study of the performance of porous ultra-thin asphalt overlay. Int. J. Pavement Eng. 2022, 23, 2049–2061. [Google Scholar] [CrossRef]
- Wu, S.; Montalvo, L. Repurposing waste plastics into cleaner asphalt pavement materials: A critical literature review. J. Clean. Prod. 2021, 280, 124355. [Google Scholar] [CrossRef]
- Jiang, W.; Xiao, J.J.; Yuan, D.D.; Lu, H.H.; Xu, S.D.; Huang, Y. Design and experiment of thermoelectric asphalt pavements with power-generation and temperature-reduction functions. Energy Build. 2018, 169, 39–47. [Google Scholar] [CrossRef]
- Fini, E.H.; Al-Qadi, I.L.; You, Z.P.; Zada, B.; Mills-Beale, J. Partial replacement of asphalt binder with bio-binder: Characterisation and modification. Int. J. Pavement Eng. 2012, 13, 515–522. [Google Scholar] [CrossRef]
- Sihombing, A.V.R.; Subagio, B.S.; Hariyadi, E.S.; Yamin, A. Mechanical Properties of Bio-Asphalt on Recycled Asphalt Pavement Binder. In Proceedings of the 9th International Conference on Maintenance and Rehabilitation of Pavements—Mairepav9, Zurich, Switzerland, 1–3 July 2020; Springer: Cham, Switzerland, 2020; pp. 529–538. [Google Scholar]
- Ren, J.L.; Zang, G.Y.; Wang, S.Y.; Shi, J.; Wang, Y.Y. Investigating the pavement performance and aging resistance of modified bio-asphalt with nano-particles. PLoS ONE 2020, 15, e0238817. [Google Scholar]
- Yan, K.Z.; Zhang, M.; You, L.Y.; Wu, S.H.; Ji, H.Y. Performance and optimization of castor beans-based bio-asphalt and European rock-asphalt modified asphalt binder. Constr. Build. Mater. 2020, 240, 117951. [Google Scholar] [CrossRef]
- Elahi, Z.; Mohd Jakarni, F.; Muniandy, R.; Hassim, S.; Ab Razak, M.S.; Ansari, A.H.; Ben Zair, M.M. Waste Cooking Oil as a Sustainable Bio Modifier for Asphalt Modification: A Review. Sustainability 2021, 13, 11506. [Google Scholar] [CrossRef]
- Jain, S.; Chandrappa, A.K. Rheological and chemical investigation on asphalt binder incorporating high recycled asphalt with waste cooking oil as rejuvenator. Innov. Infrastruct. Solut. 2022, 7, 1–19. [Google Scholar] [CrossRef]
- Zhou, X.X.; Zhao, G.Y.; Wu, S.P.; Tighe, S.; Pickel, D.; Chen, M.Z.; Adhikari, S.; Gao, Y.M. Effects of biochar on the chemical changes and phase separation of bio-asphalt under different aging conditions. J. Clean. Prod. 2020, 263, 121532. [Google Scholar] [CrossRef]
- Zhou, X.X.; Zhao, G.Y.; Miljković, M.; Tighe, S.; Chen, M.Z.; Wu, S.P. Crystallization kinetics and morphology of biochar modified bio-asphalt binder. J. Clean. Prod. 2022, 349, 131495. [Google Scholar] [CrossRef]
- Joohari, M.I.; Aziz, N.A.; Daud, N.M.; Mansor, S.; Halim, M.A. Performance of porous asphalt pavement using clay brick dust as mineral filler[C]//Journal of Physics: Conference Series. IOP Publ. 2019, 1349, 012098. [Google Scholar]
- Abdelsalam, M.; Yue, Y.C.; Khater, A.; Luo, D.; Musanyufu, J.; Qin, X.L. Laboratory study on the performance of asphalt mixes modified with a novel composite of diatomite powder and lignin fiber. Appl. Sci. 2020, 10, 5517. [Google Scholar] [CrossRef]
- Du, T.; Song, P.; Liu, L. Experimental Study on Activated Diatomite Modified Asphalt Pavement in Deep Loess Area. Processes 2022, 10, 1227. [Google Scholar] [CrossRef]
- Chen, X.L.; Sun, Y.S.; Han, Y.X.; Tian, Z.F. Microstructure and DSC of organic bentonite modified bitumen. J. Northeast. Univer. 2012, 33, 743. [Google Scholar]
- Chen, S.; Zhang, B.; He, X.Y.; Su, Y.; Liu, Q.; Xu, H. Research on mechanical-activated nanoscale bentonite and surface aging behavior of its modified asphalt. Constr. Build. Mater. 2022, 321, 126356. [Google Scholar] [CrossRef]
- Du, D.C.; Li, X.L.; Zheng, G.Y.; Zhang, L.Q. Study on the Performance of Polyester Fiber Modified Asphalt Mastic. Plastics 2012, 41, 42–45. [Google Scholar]
- Wu, S.; Ye, Q.; Li, N. Investigation of rheological and fatigue properties of asphalt mixtures containing polyester fibers. Constr. Build. Mater. 2008, 22, 2111–2115. [Google Scholar] [CrossRef]
- Cheng, C.; Sun, W.W.; Hu, B.; Tao, G.X.; Peng, C.; Tian, Y.J.; Wu, S.J. Analysis of the mechanism and effectiveness of lignin in improving the high-temperature thermal stability of asphalt. J. Renew. Mater. 2020, 8, 1243–1255. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.C.; Ji, G.Y.; Fan, Z.Y.; Guo, Y.C.; Gao, W.Z.; Xin, L. Mechanical performance characterization of lignin-modified asphalt mixture. Appl. Sci. 2020, 10, 3324. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Cai, Y.C.; Zhang, G.H.; Fang, H.Y. Fatigue property of basalt fiber-modified asphalt mixture under complicated environment. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 996–1004. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, Y.; Ma, G.; Tan, G.; Sun, X.; Yang, S. Further investigation on damage model of eco-friendly basalt fiber modified asphalt mixture under freeze-thaw cycles. Appl. Sci. 2018, 9, 60. [Google Scholar] [CrossRef]
- Arabani, M.; Shabani, A. Evaluation of the ceramic fiber modified asphalt binder. Constr. Build. Mater. 2019, 205, 377–386. [Google Scholar] [CrossRef]
- Guo, F.C.; Zhang, J.P.; Pei, J.Z.; Zhou, B.C.; Falchetto, A.C.; Hu, Z. Investigating the interaction behavior between asphalt binder and rubber in rubber asphalt by molecular dynamics simulation. Constr. Build. Mater. 2020, 252, 118956. [Google Scholar] [CrossRef]
- Guo, F.C.; Zhang, J.P.; Pei, J.Z.; Ma, W.S.; Hu, Z.; Guan, Y.S. Evaluation of the compatibility between rubber and asphalt based on molecular dynamics simulation. Front. Struct. Civ. Eng. 2020, 14, 435–445. [Google Scholar] [CrossRef]
- Zheng, W.H.; Wang, H.N.; Chen, Y.; Ji, J.; You, Z.P.; Zhang, Y.Q. A review on compatibility between crumb rubber and asphalt binder. Constr. Build. Mater. 2021, 297, 123820. [Google Scholar] [CrossRef]
- Wang, W.S.; Cheng, Y.C.; Chen, H.P.; Tan, G.J.; Lv, Z.H.; Bai, Y.S. Study on the performances of waste crumb rubber modified asphalt mixture with eco-friendly diatomite and basalt fiber. Sustainability 2019, 11, 5282. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.X.; Xiao, F.P.; Amirkhanian, S.N. Surface activation of scrap tire crumb rubber to improve compatibility of rubberized asphalt. Resour. Conserv. Recycl. 2021, 169, 105518. [Google Scholar] [CrossRef]
- Adiyanto, O.; Mohamad, E.; Abd Razak, J. Systematic Review of Plastic Waste as Eco-Friendly Aggregate for Sustainable Construction. Int. J. Sustain. Constr. Eng. Technol. 2022, 13, 243–257. [Google Scholar]
- Ben Zair, M.M.; Jakarni, F.M.; Muniandy, R.; Hassim, S. A brief review: Application of recycled polyethylene terephthalate in asphalt pavement reinforcement. Sustainability 2021, 13, 1303. [Google Scholar] [CrossRef]
- Leng, Z.; Padhan, R.K.; Sreeram, A. Production of a sustainable paving material through chemical recycling of waste PET into crumb rubber modified asphalt. J. Clean. Prod. 2018, 180, 682–688. [Google Scholar] [CrossRef]
- Qu, X.; Wang, D.W.; Wang, L.B.; Huang, Y.C.; Hou, Y.; Oeser, M. The state-of-the-art review on molecular dynamics simulation of asphalt binder. Adv. Civ. Eng. 2018, 2018, 4546191. [Google Scholar] [CrossRef]
- Yu, C.H.; Hu, K.; Chen, Y.J.; Li, Q.; Zhang, T.L.; Chen, G.X. The Influence of Styrene/butadiene Ratio on the Self-assembly Behavior of SBS Modified Asphalt[C]//IOP Conference Series: Earth and Environmental Science. IOP Publ. 2021, 668, 012060. [Google Scholar]
- Chu, L.; Luo, L.; Fwa, T.F. Effects of aggregate mineral surface anisotropy on asphalt-aggregate interfacial bonding using molecular dynamics (MD) simulation. Constr. Build. Mater. 2019, 225, 1–12. [Google Scholar] [CrossRef]
- Hu, B.; Huang, W.K.; Yu, J.L.; Xiao, Z.C.; Wu, K.H. Study on the adhesion performance of asphalt-calcium silicate hydrate gel interface in semi-flexible pavement materials based on molecular dynamics. Materials 2021, 14, 4406. [Google Scholar] [CrossRef]
- Hu, D.L.; Pei, J.Z.; Li, R.; Zhang, J.P.; Jia, Y.S.; Fan, Z.P. Using thermodynamic parameters to study self-healing and interface properties of crumb rubber modified asphalt based on molecular dynamics simulation. Front. Struct. Civ. Eng. 2020, 14, 109–122. [Google Scholar] [CrossRef]
- Yan, L.M.; Zhu, S.H. Theory and Practice of Molecular Dynamics Simulation; Science Press: Beijing, China, 2013. [Google Scholar]
- Sun, H.; Rigby, D. Polysiloxanes: Ab initio force field and structural, conformational and thermophysical properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1997, 53, 1301–1323. [Google Scholar] [CrossRef]
- Sun, H. An ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. J. Phys. Chem. 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Sun, H.; Ren, P.; Fried, J.R. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Bunte, S.W.; Sun, H. Molecular modeling of energetic materials: The parameterization and validation of nitrate esters in the COMPASS force field. J. Phys. Chem. B 2000, 104, 2477–2489. [Google Scholar] [CrossRef]
- Sun, H.; Jin, Z.; Yang, C.W.; Akkermans, R.L.C.; Robertson, S.H.; Spenley, N.A.; Miller, S.; Todd, S.M. COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Model. 2016, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Su, M.M.; Si, C.D.; Zhang, Z.P.; Zhang, H.L. Molecular dynamics study on influence of Nano-ZnO/SBS on physical properties and molecular structure of asphalt binder. Fuel 2020, 263, 116777. [Google Scholar] [CrossRef]
- Zhang, Y. Fundamentals of Computational Materials Science; Beijing University of Aeronautics and Astronautics Press: Beijing, China, 2007. [Google Scholar]
- Lan, Y.H.; Li, D.H.; Yang, R.J.; Liang, W.S.; Zhou, L.X.; Chen, Z.W. Computer simulation study on the compatibility of cyclotriphosphazene containing aminopropylsilicone functional group in flame retarded polypropylene/ammonium polyphosphate composites. Compos. Sci. Technol. 2013, 88, 9–15. [Google Scholar] [CrossRef]
- D’hooge, D.R.; Van Steenberge, P.H.M.; Reyniers, M.F.; Marin, G.B. The strength of multi-scale modeling to unveil the complexity of radical polymerization. Prog. Polym. Sci. 2016, 58, 59–89. [Google Scholar] [CrossRef]
- Hu, K.; Yu, C.H.; Chen, Y.J.; Li, W.; Wang, D.D.; Zhang, W.G. Multiscale mechanisms of asphalt performance enhancement by crumbed waste tire rubber: Insight from molecular dynamics simulation. J. Mol. Model. 2021, 27, 1–14. [Google Scholar] [CrossRef]









| Components | Molecular Formula | Number Ratio | Relative Molecular Mass | Mass Fraction (%) | NumAtoms |
|---|---|---|---|---|---|
| Asphaltene | C149H177N3O2S2 | 1 | 2106.19 | 19.734 | 333 |
| Resin | C59H85NOS | 3 | 856.395 | 24.071 | 147 |
| Saturate | C22H46 | 5 | 310.610 | 14.551 | 68 |
| Aromatic | C46H50S | 7 | 634.966 | 41.645 | 97 |
| Serial Number | Representative Molecule | Chemical Composition | Molecule Number | Relative Molecular Mass | Mass Fraction |
|---|---|---|---|---|---|
| 1 | Asphaltene | C149H177N3O2S2 | 1 | 2106.190 | 17.90% |
| 2 | Resin | C59H85NOS | 3 | 856.395 | 21.84% |
| 3 | Saturate | C22H46 | 5 | 310.610 | 13.20% |
| 4 | Aromatic | C46H50S | 7 | 634.966 | 37.78% |
| 5 | NR | C80 H130 | 1 | 1091.920 | 9.28% |
| Serial Number | Representative Molecule | Chemical Composition | Molecule Number | Relative Molecular Mass | Mass Fraction |
|---|---|---|---|---|---|
| 1 | Asphaltene | C149H177N3O2S2 | 1 | 2106.190 | 17.40% |
| 2 | Resin | C59H85NOS | 3 | 856.395 | 21.23% |
| 3 | Saturate | C22H46 | 5 | 310.610 | 12.83% |
| 4 | Aromatic | C46H50S | 7 | 634.966 | 36.72% |
| 5 | NR | C80 H130 | 1 | 1091.920 | 9.02% |
| 6 | PE | C24H50 | 1 | 338.664 | 2.80% |
| Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polymerization degree | 6 | 12 | 18 | 24 | 30 | 36 | 42 | 48 | 54 | 60 | 66 | 72 | 78 | 84 |
| Solubility parameter | 17.85 | 17.09 | 16.66 | 15.66 | 14.33 | 15.56 | 14.99 | 15.71 | 15.47 | 16.08 | 14.70 | 15.92 | 15.79 | 15.02 |
| Equation | |||||
|---|---|---|---|---|---|
| Plot | asphaltene | resin | saturate | aromatic | NR |
| Weight | No Weighting | ||||
| Intercept | 1.68015 ± 0.14844 | 1.55818 ± 0.1093 | 2.44376 ± 0.15638 | 1.38694 ± 0.02799 | 2.007 ± 0.06071 |
| Slope | 0.01035 ± 0.0029 | 0.01188 ± 0.00213 | 0.12047 ± 0.00305 | 0.02361 ± 5.46305E-4 | 0.01028 ± 0.00118 |
| Residual Sum of Squares | 1.28424 | 0.69631 | 1.42537 | 0.04566 | 0.21482 |
| Pearson’s r | 0.67805 | 0.82105 | 0.99522 | 0.99601 | 0.91308 |
| R-Square (COD) | 0.45975 | 0.67412 | 0.99046 | 0.99204 | 0.83371 |
| Adj. R-Square | 0.42373 | 0.65239 | 0.98983 | 0.9915 | 0.82263 |
| Equation | |||||
|---|---|---|---|---|---|
| Plot | asphaltene | resin | saturate | aromatic | NR |
| Weight | No Weighting | ||||
| Intercept | 1.61155 ± 0.11877 | 1.75603 ± 0.12832 | 2.74225 ± 0.25882 | 1.53794 ± 0.05243 | 2.10403 ± 0.06517 |
| Slope | 0.02598 ± 0.00232 | 0.07596 ± 0.0025 | 0.14313 ± 0.00505 | 0.03484 ± 0.00102 | 0.0455 ± 0.00127 |
| Residual Sum of Squares | 0.82214 | 0.95978 | 3.90458 | 0.1602 | 0.24752 |
| Pearson’s r | 0.94514 | 0.99194 | 0.99079 | 0.99359 | 0.99419 |
| R-Square (COD) | 0.89329 | 0.98395 | 0.98166 | 0.98722 | 0.98842 |
| Adj. R-Square | 0.88618 | 0.98288 | 0.98044 | 0.98637 | 0.98764 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Hu, K.; Yu, C.; Yuan, D.; Ban, X. Study of the Microscopic Mechanism of Natural Rubber (Cis-1, 4-Polyisoprene, NR)/Polyethylene (PE) Modified Asphalt from the Perspective of Simulation. Polymers 2022, 14, 4087. https://doi.org/10.3390/polym14194087
Chen Y, Hu K, Yu C, Yuan D, Ban X. Study of the Microscopic Mechanism of Natural Rubber (Cis-1, 4-Polyisoprene, NR)/Polyethylene (PE) Modified Asphalt from the Perspective of Simulation. Polymers. 2022; 14(19):4087. https://doi.org/10.3390/polym14194087
Chicago/Turabian StyleChen, Yujing, Kui Hu, Caihua Yu, Dongdong Yuan, and Xiaoyi Ban. 2022. "Study of the Microscopic Mechanism of Natural Rubber (Cis-1, 4-Polyisoprene, NR)/Polyethylene (PE) Modified Asphalt from the Perspective of Simulation" Polymers 14, no. 19: 4087. https://doi.org/10.3390/polym14194087
APA StyleChen, Y., Hu, K., Yu, C., Yuan, D., & Ban, X. (2022). Study of the Microscopic Mechanism of Natural Rubber (Cis-1, 4-Polyisoprene, NR)/Polyethylene (PE) Modified Asphalt from the Perspective of Simulation. Polymers, 14(19), 4087. https://doi.org/10.3390/polym14194087