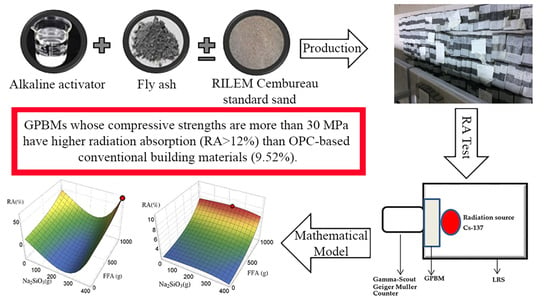
Eco-Geopolymers: Physico-Mechanical Features, Radiation Absorption Properties, and Mathematical Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials Characteristic
2.2. GPBM Assessment Methods
3. Results and Discussion
3.1. The Properties of Raw Materials
3.2. GPBMs Production and Properties
3.3. Optimization Methods, Curve Fitting, and Mathematical Modeling
4. Conclusions
- (1)
- FFAs with total aggregate content of 70–80%, 12 M NaOH, the Na2SiO3/NaOH ratio of 1–2.5, and 24 h of curing at 70 or 100 °C all represent the conditions for GPBM production that result in final materials with an average compressive strength of 40–44 and 58–63 MPa for the GPBM produced from the Catalagzi TPP and Isken TPP FFAs, respectively.
- (2)
- Higher reactivity of the Isken TPP FFA, and thus better mechanical and physical properties of the geopolymer, resulted from finer particles and greater surface area of raw material. The highest compressive strength was measured as 93.3 MPa for the GPBM produced with 10% NaOH and cured at 100 °C.
- (3)
- The best GPBM (produced from the Isken TPP FFA) had the highest RA of 12.5%, density of 1.70 g cm−3, porosity of 19.9%, water absorption of 12.4%, and compressive strength of 57.3 MPa; thus, eco-friendly GPBMs are lightweight construction materials with good mechanical properties.
- (4)
- According to the mathematical model developed in this study, the effect of FFA/alkali activator type and quantity on RA is an important issue. Optimization is required to obtain maximum RA values. Mathematical modeling and appropriate algorithms can provide this without costly experimentation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okoye, F.N.; Durgaprasad, J.; Singh, N.B. Effect of silica fume on the mechanical properties of fly ash based-geopolymer concrete. Ceram. Int. 2016, 42, 3000–3006. [Google Scholar] [CrossRef]
- Korniejenko, K.; Łach, M.; Dogan-Saglamtımur, N.; Furtos, G.; Mikuła, J. The overview of mechanical properties of short natural fiber reinforced geopolymer composites. Environ. Res. Technol. 2020, 3, 28–39. [Google Scholar] [CrossRef]
- Luhar, S.; Luhar, I. Fly ash based geopolymer mortar-strength performance. Int. J. Recent Technol. Eng. (IJRTE) 2020, 8, 1181–1186. [Google Scholar]
- Szechyńska-Hebda, M.; Marczyk, J.; Ziejewska, C.; Hordyńska, N.; Mikuła, J.; Hebda, M. Neutral geopolymer foams reinforced with cellulose studied with the FT-Raman spectroscopy. IOP Conf. Ser. Mater. Sci. Eng. 2019, 706, 012017. [Google Scholar] [CrossRef]
- Doğan-Sağlamtimur, N.; Bilgil, A.; Öztürk, B. Reusability of Ashes for the Building Sector to Strengthen the Sustainability of Waste Management. In Handbook of Research on Supply Chain Management for Sustainable Development; IGI Global Publishing: Hershey, PA, USA, 2018; Chapter 14; pp. 265–281. ISBN 9781522557579. [Google Scholar] [CrossRef]
- Kurtoğlu, A.E.; Alzeebaree, R.; Aljumaili, O.; Niş, A.; Gülşan, M.E.; Humur, G.; Çevik, A. Mechanical and durability properties of fly ash and slag based geopolymer concrete. Adv. Concr. Constr. 2018, 6, 345–362. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Ghoshal, S.K.; Hussein, A.A. Geopolymer mortars as sustainable repair material: A comprehensive review. Renew. Sust. Energ. Rev. 2017, 80, 54–74. [Google Scholar] [CrossRef]
- Gado, R.A.; Hebda, M.; Łach, M.; Mikuła, J. Alkali Activation of Waste Clay Bricks: Influence of The Silica Modulus, SiO2/Na2O, H2O/Na2O Molar Ratio, and Liquid/Solid Ratio. Materials 2020, 13, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, B.; Zhao, Y.; Bai, H.; Kang, S.; Zhang, T.; Song, S. Eco-friendly geopolymer prepared from solid wastes: A critical review. Chemosphere 2021, 267, 128900. [Google Scholar] [CrossRef]
- Doğan-Sağlamtimur, N.; Bilgil, A.; Szechyńska-Hebda, M.; Parzych, S.; Hebda, M. Eco-Friendly Fired Brick Produced from Industrial Ash and Natural Clay: A Study of Waste Reuse. Materials 2021, 14, 877. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y. Zheng, Z.; Deng, Y.; Shi, C.; Zhang, Z. Advances in immobilization of radionuclide wastes by alkali activated cement and related materials. Cem. Concr. Comp. 2022, 126, 104377. [Google Scholar] [CrossRef]
- Asim, N.; Alghoul, M.; Mohammad, M.; Amin, M.H.; Akhtaruzzaman, M.; Amin, N.; Sopian, K. Emerging sustainable solutions for depollution: Geopolymers. Constr. Build. Mater. 2019, 199, 540–548. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Marczyk, J.; Ziejewska, C.; Hordyńska, N.; Mikuła, J.; Hebda, M. Optimal Design of pH-neutral Geopolymer Foams for Their Use in Ecological Plant Cultivation Systems. Materials 2019, 12, 2999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidovits, J. SPE PACTEC’79; Society of Plastic Engineers: Danbury, CT, USA, 1979. [Google Scholar]
- Koumoto, T. Production of high compressive strength geopolymers considering fly ash or slag chemical composition. J. Mater. Civ. Eng. 2019, 31, 06019006. [Google Scholar] [CrossRef] [Green Version]
- Djobo, J.N.Y.; Elimbi, A.; Tchakouté, H.K.; Kumar, S. Volcanic ash-based geopolymer cements/concretes: The current state of the art and perpectives. Environ. Sci. Pollut. Res. 2017, 24, 4433–4446. [Google Scholar] [CrossRef] [PubMed]
- Öz, H.Ö.; Doğan-Sağlamtimur, N.; Bilgil, A.; Tamer, A.; Günaydın, K. Process Development of Fly Ash-Based Geopolymer Mortars in view of Mechanical Characteristics. Materials 2021, 14, 2935. [Google Scholar] [CrossRef] [PubMed]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Arnoult, M.; Perronnet, M.; Autef, A.; Rossignol, R. How to control the geopolymer setting time with the alkaline silicate solution. J. Non-Cryst Solids. 2018, 495, 59–66. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; De Silva, P.; Hanjitsuwan, S. Effect of high-speed mixing on properties of high calcium fly ash geopolymer paste. Arab. J. Sci. Eng. 2014, 39, 6001–6007. [Google Scholar] [CrossRef]
- Marczyk, J.; Ziejewska, C.; Gądek, S.; Korniejenko, K.; Łach, M.; Góra, M.; Kurek, I.; Doğan-Sağlamtimur, N.; Hebda, M.; Szechyńska-Hebda, M. Hybrid Materials Based on Fly Ash, Metakaolin, and Cement for 3D Printing. Materials 2021, 14, 6874. [Google Scholar] [CrossRef]
- Mohammed, K.S.; Azeez, A.B.; Al Bakri, A.M.M.; Hussin, K.; Rahmat, A.B. The effect of barite content on anti radiation properties of geopolymer fly ash concrete incorporated natural rock ores of hematite. Int. J. Sci. Res. 2014, 3, 1818–1827. [Google Scholar]
- Aygün, B. Neutron and gamma radiation shielding properties of high-temperature-resistant heavy concretes including chromite and wolframite. J. Radiat. Res. Appl. Sci. 2019, 12, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Akkurt, I.; Akyıldırım, H.; Mavi, B.; Kılınçarslan, Ş.; Basyigit, C. The effect of pumice rate on the gamma absorption parameters of concrete. Acta Phys. Pol. A 2012, 121, 144–146. [Google Scholar] [CrossRef]
- Adewuyi, Y.G. Recent Advances in Fly-Ash-Based Geopolymers: Potential on the Utilization for Sustainable Environmental Remediation. ACS Omega 2021, 6, 15532–15542. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, V.; Motooka, T.; Yamagishi, I. Geopolymers and Their Potential Applications in the Nuclear Waste Management Field-A Bibliographical Study-Japan Atomic Energy Agency; JAEA-Review 2017-014; International Atomic Energy Agency (IAEA): Vienna, Austria, 2017. [Google Scholar]
- Jang, J.G.; Park, S.M.; Lee, H.K. Physical barrier effect of geopolymeric waste form on diffusivity of cesium and strontium. J. Hazard. Mater. 2016, 318, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, J.; Kang, J.; Um, W. Development of geopolymer waste form for immobilization of radioactive borate waste. J. Hazard. Mater. 2021, 419, 126402. [Google Scholar] [CrossRef]
- Shalbi, S.M.; Jaafar, M.S.; Ahmed, N.M.; Al-Jarrah, A.M.; Naji, A.; Alsadig Ahmed, A.; Qaeed, M.A. Effect of fly ash geopolymer with 15% barium sulphate as a design shielding box on radiation attenuation using GafchramicXR-QA2 film dosimetry. IOSR J. Eng. (IOSRJEN) 2017, 7, 13–17. [Google Scholar] [CrossRef]
- Kubissa, W.; Glinicki, M.A.; Dąbrowski, M. Permeability testing of radiation shielding concrete manufactured at industrial scale. Mater. Struct. 2018, 51, 83. [Google Scholar] [CrossRef] [Green Version]
- Basyigit, C.; Uysal, V.; Kilincarslan, Ş.; Mavi, B.; Günoğlu, K.; Akkurt, I.; Akkaş, A. Investigating radiation shielding properties of different mineral origin heavyweight concretes. AIP Conf. Proc. 2011, 1400, 232–235. [Google Scholar] [CrossRef]
- The International Atomic Energy Agency. The Behaviours of Cementitious Materials in Long Term Storage and Disposal of Radioactive Waste IAEA-TECDOC-1701; International Atomic Energy Agency (IAEA): Vienna, Austria, 2013. [Google Scholar]
- Tamzok, N. Domestic Coal-based Thermal Power Plant. Potential, Constraints and Solutions, Thermal Power Plants in Turkey (Yerli Kömüre Dayalı Termik Santral Potansiyeli, Darboğazlar ve Çözüm Önerileri, Türkiye’de Termik Santraller); TMMOB Publication Number: Ankara, Turkey, 2017; pp. 135–142. [Google Scholar]
- Özkan, A.; Turan, E.; Kaplan, B.M. Investigation of fly ash effect on rheological and filtration properties of drilling muds. Fresenius Environ. Bull. 2018, 27, 9189–9194. [Google Scholar]
- TS EN 933-10 Tests for Geometrical Properties of Aggregates-Part 10: Assessment of Fines-Grading of Filler Aggregates (Air Jet Sieving), In Turkish Standards. Available online: https://standards.iteh.ai/catalog/standards/cen/cc9acb3e-3176-4461-8e2d-6cb1dfc816e7/en-933-10-2009 (accessed on 8 January 2022).
- Arıcı, E.; Çelik, E.; Keleştemur, O. An analysis of the engineering properties of mortars containing corn cob ash and polypropylene fiber using the Taguchi and Taguchi-based Grey Relational Analysis methods. Case Stud. Constr. Mater. 2021, 15, e00652. [Google Scholar] [CrossRef]
- ASTM C1437-15 Standard Test Method for Flow of Hydraulic Cement Mortar. In ASTM International. Available online: https://www.astm.org/c1437-15.html (accessed on 8 January 2022).
- TS EN 772-3 Methods of Test for Masonry Units-Part 3: Determination of Net Volume and Percentage of Voids of Clay Masonry Units by Hydrostatic Weighing; Türk Standardları Enstitüsü (Turkish Standards Institution): Ankara, Turkey. Available online: https://standards.iteh.ai/catalog/standards/cen/724fb5fe-662d-40cf-b5b1-cc348aa45ca6/en-772-3-1998 (accessed on 8 January 2022).
- ASTM C348-14 Standard Test Method for Flexural Strength of Hydraulic-Cement Mortars. In ASTM International. Available online: https://www.astm.org/c0348-14.html (accessed on 8 January 2022).
- ASTM C349-14 Standard Test Method for Compressive Strength of Hydraulic-Cement Mortars (Using Portions of Prisms Broken in Flexure). In ASTM International. Available online: https://www.astm.org/c0349-14.html (accessed on 8 January 2022).
- TS EN 12390-3 Testing Hardened Concrete-Part 3: Compressive Strength of Test Specimens. Available online: http://www.vota.com.tr/assets/ts-en-12390-3.pdf (accessed on 8 January 2022).
- Diaz, E.I.; Allouche, E.N.; Eklund, S. Factors affecting the suitability of fly ash as source material for geopolymers. Fuel 2010, 89, 992–996. [Google Scholar] [CrossRef]
- Valentim, B.V.; Hower, J.C. Influence of feed and sampling systems on element partitioning in Kentucky fly ash. Int J. Coal Geol. 2010, 82, 94–104. [Google Scholar] [CrossRef]
- Łach, M.; Gado, R.A.; Marczyk, J.; Ziejewska, C.; Dogan-Saglamtimur, N.; Mikuła, J.; Szechyńska-Hebda, M.; Hebda, M. Process design for a production of sustainable materials from post-production clay. Materials 2021, 14, 953. [Google Scholar] [CrossRef]
- ASTM C618 Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use as a Mineral Admixture in Concrete, In ASTM International. Available online: https://www.astm.org/c0618-00.html (accessed on 8 January 2022).
- Osholana, T.S.; Dludlu, M.K.; Oboirien, B.; Sadiku, R. Enhanced reactivity of geopolymers produced from fluidized bed combustion bottom ash. S. Afr. J. Chem. Eng. 2020, 34, 72–77. [Google Scholar] [CrossRef]
- Bhatt, A.; Priyadarshini, S.; Mohanakrishnan, A.A.; Abri, A.; Sattler, M.; Techapaphawit, S. Physical, chemical, and geotechnical properties of coal fly ash: A global review. Case Stud. Constr. Mater. 2019, 11, e00263. [Google Scholar] [CrossRef]
- TS EN 196-1 Methods of Testing Cement-Part 1: Determination of Strength, In Turkish Standards. Available online: https://standards.iteh.ai/catalog/standards/cen/37b8816e-4085-4dcc-a642-a383d9bddd6c/en-196-1-2016 (accessed on 8 January 2022).
- Nath, S.K.; Kumar, S. Role of particle fineness on engineering properties and microstructure of fly ash derived geopolymer. Constr. Build. Mater. 2020, 233, 117294. [Google Scholar] [CrossRef]
- Firdaus, Y.İ.; Daud, R. Contribution of fineness level of fly ash to the compressive strength of geopolymer mortar. MATEC Web Conf. 2017, 103, 01026. [Google Scholar] [CrossRef]
- Jamkar, S.S.; Ghugal, Y.M.; Patankar, S.V. Effect of fineness of fly ash fineness on workability and compressive strength of geopolymer concrete. Indian Concr. J. 2013, 87, 57–61. [Google Scholar]
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Demirel, Y. An experimental purpose for correlation of data of rebound hammer as to axial load levels on the different strength of concrete columns. Düzce Üniversitesi Bilim ve Teknol. Dergisi. 2015, 3, 76–87. [Google Scholar]
- Yalcin, M. Building Material/Application; Eskisehir, Turkey. 2021. Available online: https://insaat.eskisehir.edu.tr/muhsiny/MLZ204/icerik/12H-2)%20Beton%20üretimi-taze%20beton%20deneyleri-2-1%20(2020-2021).pdf (accessed on 8 January 2022). (In Turkish).
- Mermerdaş, K.; Manguri, S.; Nassani, D.E.; Oleiwi, S.M. Effect of aggregate properties on the mechanical and absorption characteristics of geopolymer mortar. Eng. Sci. Technol. Int. J. 2017, 20, 1642–1652. [Google Scholar] [CrossRef]
- Mierzwiński, D.; Łach, M.; Hebda, M.; Walter, J.; Szechyńska-Hebda, M.; Mikuła, J. Thermal phenomena of alkali-activated metakaolin studied with a negative temperature coefficient system. J. Therm Anal. Calorim. 2019, 138, 4167–4175. [Google Scholar] [CrossRef] [Green Version]
- Korniejenko, K.; Figiela, B.; Miernik, K.; Ziejewska, C.; Marczyk, J.; Hebda, M.; Cheng, A.; Lin, W.-T. Mechanical and Fracture Properties of Long Fiber Reinforced Geopolymer Composites. Materials 2021, 14, 5183. [Google Scholar] [CrossRef]
- Leay, L.; Potts, A.; Donoclift, T. Geopolymers from fly ash and their gamma irradiation. Mater. Lett. 2018, 227, 240–242. [Google Scholar] [CrossRef]
- Uzun, I. Numerical Analysis (in Turkish); Beta Publishing: İstanbul, Turkey, 2011. [Google Scholar]
- Champion, B.; Strzebonski, A. Constrained Optimization; Wolfram Mathematica Tutorial Collection: Champaign, IL, USA, 2008. [Google Scholar]
- Tyagi, G.; Singhal, A.; Routroy, S.; Bhunia, D.; Lahoti, M. Radiation Shielding Concrete with alternate constituents: An approach to address multiple hazards. J. Hazard. Mater. 2021, 404, 124201. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tang, Q.; Cui, X.; He, Y.; Liu, L. Development of near-zero water consumption cement materials via the geopolymerization of tektites and its implication for lunar construction. Sci. Rep.-UK 2016, 6, 29659. [Google Scholar]
- Montes, C.; Broussard, K.; Gongre, M.; Simicevic, N.; Mejia, J.; Tham, J.; Allouche, E.; Davis, G. Evaluation of lunar regolith geopolymer binder as a radioactive shielding material for space exploration applications. Adv. Space Res. 2015, 56, 1212–1221. [Google Scholar] [CrossRef]




| FFA Type | NaOH to Na2SiO3 Ratio | Sand | Alkaline Solution to FFA (% by Weight) | Curing Temperature |
|---|---|---|---|---|
| Isken TPP, Catalagzi TPP | 1:0 | + | 10% | 70 °C, 100 °C |
| 1:0 | - | 15%, 25% | ||
| 1:1 | + | 10% | ||
| 1:1 | - | 10% | ||
| 1:1.5 | + | 10%, 20% | ||
| 1:1.5 | - | 10%, 20% | ||
| 1:2 | + | 10%, 20% | ||
| 1:2 | - | 10%, 20% | ||
| 1:2.5 | + | 20% | ||
| 1:2.5 | - | 20% |
| FFA Type | SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | MgO | K2O | SO3 | Other Oxides | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| Catalagzi TPP | 54.08 | 26.08 | 6.68 | 2.00 | 0.79 | 2.67 | 4.53 | 0.73 | 2.44 | 1.52 |
| Isken TPP | 62.28 | 21.46 | 7.01 | 1.53 | 0.26 | 2.37 | 3.81 | 0.07 | 1.21 | 1.78 |
| Properties | Catalagzi TPP FFA | Isken TPP FFA |
|---|---|---|
| BET (m2 g−1) | 1.11 | 2.26 |
| Specific gravity | 2.04 | 2.25 |
| Air-dried loose bulk density (g cm−3) | 0.87 | 1.10 |
| Air-dried tight bulk density (g cm−3) | 1.04 | 1.14 |
| Oven-dried loose bulk density (g cm−3) | 0.75 | 0.98 |
| Oven-dried tight bulk density (g cm−3) | 0.88 | 1.05 |
 | Diameter of Sand Grain | 0.08 | 0.16 | 0.5 | 1.0 | 1.6 | 2.0 |
| Remaining (%) | 99 | 87 | 72 | 34 | 6 | 0 | |
| Limits of specification (%) | 99 ± 1 | 99 ± 5 | 67 ± 5 | 33 ± 5 | 7 ± 5 | 0 |
| Mixing Ratio of Raw Materials | GPBM Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample No | NaOH (g) | Na2SiO3 (g) | Alkaline Solution to FFA (% by Weight) | Density (g cm−3) | Porosity (%) | Water Absorption (%) | Flexural Strength (MPa) | Compressive Strength (MPa) | Radiation Absorption (%) |
| GPBMs produced from Catalagzi TPP FFA | |||||||||
| Curing temperature 70 °C | |||||||||
| 1 | 300 | - | 15* | 1.46 | 30.20 | 22.30 | 5.3 | 30.3 | 3.00 |
| 2 | 160 | 320 | 20 | 1.84 | 28.94 | 17.98 | 6.1 | 33.5 | 12.36 |
| 3 | 120 | 120 | 10 | 1.69 | 23.65 | 14.81 | 7.8 | 39.0 | 2.56 |
| 4 | 80 | 160 | 10 | 1.79 | 25.42 | 16.38 | 8.7 | 47.3 | 2.12 |
| 5 | 134 | 266 | 20* | 1.46 | 21.91 | 15.63 | 4.6 | 53.0 | 2.38 |
| Curing temperature 100 °C | |||||||||
| 6 | 96 | 144 | 10 | 1.56 | 25.23 | 16.56 | 8.2 | 34.2 | 2.56 |
| 7 | 80 | 160 | 10 | 1.53 | 20.45 | 13.29 | 8.9 | 40.8 | 2.20 |
| 8 | 160 | 320 | 20 | 1.58 | 25.23 | 16.56 | 3.9 | 46.1 | 3.88 |
| 9 | 500 | - | 25* | 1.45 | 31.23 | 23.75 | 3.9 | 47.0 | 2.03 |
| 10 | 160 | 240 | 20* | 1.24 | 23.40 | 17.50 | 3.0 | 47.5 | 3.53 |
| 11 | 114 | 286 | 20* | 1.32 | 22.80 | 17.46 | 8.8 | 49.0 | 5.21 |
| GPBMs produced from Isken TPP FFA | |||||||||
| Curing temperature 70 °C | |||||||||
| 12 | 174 | 346 | 20 | 1.94 | 28.94 | 17.98 | 13.1 | 46.9 | 0.88 |
| 13 | 500 | - | 25* | 1.69 | 28.85 | 18.34 | 1.50 | 47.0 | 3.35 |
| 14 | 330 | - | 15* | 1.81 | 26.69 | 16.46 | 7.35 | 63.5 | 5.91 |
| 15 | 125 | 315 | 20* | 1.84 | 23.83 | 14.80 | 6.90 | 66.6 | 7.24 |
| 16 | 130 | 130 | 10 | 2.00 | 26.84 | 17.55 | 13.4 | 71.4 | 5.21 |
| 17 | 80 | 160 | 10 | 2.04 | 18.92 | 9.94 | 18.3 | 81.4 | 4.24 |
| Curing temperature 100 °C | |||||||||
| 18 | 110 | 110 | 10* | 1.67 | 25.64 | 16.57 | 6.1 | 37.1 | 6.71 |
| 19 | 148 | 372 | 20 | 1.98 | 16.16 | 10.07 | 4.2 | 46.6 | 0.61 |
| 20 | 177 | 351 | 20 | 1.97 | 17.94 | 9.72 | 13.3 | 49.6 | 4.85 |
| 21 | 330 | - | 15* | 1.69 | 23.09 | 14.45 | 16.2 | 52.4 | 4.68 |
| 22 | 125 | 315 | 20* | 1.70 | 19.91 | 12.39 | 3.0 | 57.3 | 12.54 |
| 23 | 87 | 173 | 10 | 1.95 | 20.88 | 13.24 | 6.8 | 60.1 | 2.12 |
| 24 | 106 | 158 | 10 | 1.92 | 16.48 | 8.44 | 16.7 | 65.5 | 10.15 |
| 25 | 264 | - | 10 | 2.01 | 13.94 | 7.09 | 16.5 | 93.3 | 4.77 |
| Optimization Method * | GPBMs | NaOH (g) | Na2SiO3 (g) | FFA (g) | Standard Sand (g) | RA (%) |
|---|---|---|---|---|---|---|
| SA DE | GPBMCTPP-70 | 0 | 400 | 1000 | 200 | 70.7 |
| GPBMCTPP-100 | 0 | 400 | 1000 | 200 | 13.9 | |
| GPBMITPP-70 | 202 | 222 | 1000 | 200 | 8.0 | |
| GPBMITPP-100 | 169 | 194 | 1000 | 200 | 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doğan-Sağlamtimur, N.; Bilgil, A.; Ertürk, S.; Bozkurt, V.; Süzgeç, E.; Akan, A.G.; Nas, P.; Çetin, H.; Szechyńska-Hebda, M.; Hebda, M. Eco-Geopolymers: Physico-Mechanical Features, Radiation Absorption Properties, and Mathematical Model. Polymers 2022, 14, 262. https://doi.org/10.3390/polym14020262
Doğan-Sağlamtimur N, Bilgil A, Ertürk S, Bozkurt V, Süzgeç E, Akan AG, Nas P, Çetin H, Szechyńska-Hebda M, Hebda M. Eco-Geopolymers: Physico-Mechanical Features, Radiation Absorption Properties, and Mathematical Model. Polymers. 2022; 14(2):262. https://doi.org/10.3390/polym14020262
Chicago/Turabian StyleDoğan-Sağlamtimur, Neslihan, Ahmet Bilgil, Sefa Ertürk, Vakkas Bozkurt, Elif Süzgeç, Arife Gözde Akan, Pervin Nas, Hüseyin Çetin, Magdalena Szechyńska-Hebda, and Marek Hebda. 2022. "Eco-Geopolymers: Physico-Mechanical Features, Radiation Absorption Properties, and Mathematical Model" Polymers 14, no. 2: 262. https://doi.org/10.3390/polym14020262
APA StyleDoğan-Sağlamtimur, N., Bilgil, A., Ertürk, S., Bozkurt, V., Süzgeç, E., Akan, A. G., Nas, P., Çetin, H., Szechyńska-Hebda, M., & Hebda, M. (2022). Eco-Geopolymers: Physico-Mechanical Features, Radiation Absorption Properties, and Mathematical Model. Polymers, 14(2), 262. https://doi.org/10.3390/polym14020262