Microencapsulation of Curcumin in Crosslinked Jelly Fig Pectin Using Vacuum Spray Drying Technique for Effective Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extraction of Pectin from Jelly Fig Achenes
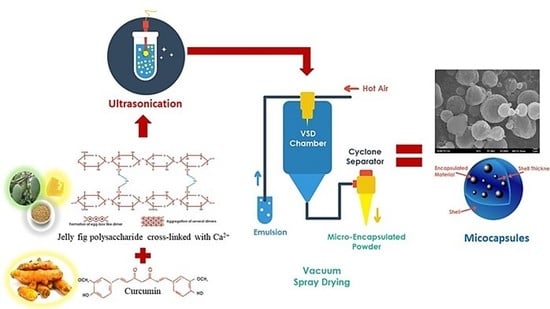
2.2.2. Curcumin-Jelly Fig Pectin Microcapsules (C-JFMs) Production
2.2.3. Size Distribution and Viscosity of the Emulsion
2.2.4. Yield and Moisture Content of C-JFMs
2.2.5. Drug Loading Efficiency (LE) and Encapsulation Efficiency (EE)
2.2.6. The Characterization of C-JFMs
2.2.7. Antioxidant Stability during Storage
2.2.8. In Vitro Release Study
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Particle Size Distribution and Viscosity of the Emulsion
3.2. The Yield and Moisture Content of Microcapsules
3.3. Drug Loading Efficiency (LE) and Encapsulation Efficiency (EE)
3.4. Morphology and Particle Size Distribution
3.5. Fourier Transform Infrared (FTIR) Spectroscopy
3.6. Thermogravimetric Analysis (TGA)
3.7. Antioxidant Stability during Storage
3.8. In Vitro Release Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hoyos-Leyva, J.; Bello-Perez, L.; Agama-Acevedo, J.; Alvarez-Ramirez, J.; Jaramillo-Echeverry, L.J.L. Characterization of spray drying microencapsulation of almond oil into taro starch spherical aggregates. LWT 2019, 101, 526–533. [Google Scholar] [CrossRef]
- Santa-Maria, M.; Scher, H.; Jeoh, T. Microencapsulation of bioactives in cross-linked alginate matrices by spray drying. J. Microencapsul. 2012, 29, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Strobel, S.A.; Scher, H.B.; Nitin, N.; Jeoh, T. In situ cross-linking of alginate during spray-drying to microencapsulate lipids in powder. J. Microencapsul. 2016, 58, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Cameron, R.G.; Bai, J. Effect of spray-drying temperature on physicochemical, antioxidant and antimicrobial properties of pectin/sodium alginate microencapsulated carvacrol. Food Hydrocoll. 2020, 100, 105420. [Google Scholar] [CrossRef]
- Risch, S.J. Encapsulation: Overview of Uses and Techniques; ACS Publications: Washington, DC, USA, 1995. [Google Scholar]
- do Amaral, P.H.R.; Andrade, P.L.; de Conto, L.C. Microencapsulation and Its Uses in Food Science and Technology: A review. Microencapsul. Process. Technol. Ind. Appl. 2019, 93. [Google Scholar] [CrossRef] [Green Version]
- Veiga, R.D.S.D.; Aparecida Da Silva-Buzanello, R.; Corso, M.P.; Canan, C. Essential oils microencapsulated obtained by spray drying: A review. J. Essent. Oil Res. 2019, 31, 457–473. [Google Scholar] [CrossRef]
- Bamidele, O.P.; Emmambux, M.N. Encapsulation of bioactive compounds by “extrusion” technologies: A review. Crit. Rev. Food Sci. Nutr. 2020, 1–19. [Google Scholar] [CrossRef] [PubMed]
- de Melo Ramos, F.; Júnior, V.S.; Prata, A.S. Impact of vacuum spray drying on encapsulation of fish oil: Oxidative stability and encapsulation efficiency. Food Res. Int. 2021, 143, 110283. [Google Scholar] [CrossRef] [PubMed]
- Chiao, C.; Price, J.C. Formulation, preparation and dissolution characteristics of propranolol hydrochloride microspheres. J. Microencapsul. 1994, 11, 153–159. [Google Scholar] [CrossRef]
- de Melo Ramos, F.; Ubbink, J.; Júnior, V.S.; Prata, A.S. Drying of Maltodextrin solution in a vacuum spray dryer. Chem. Eng. Res. Des. 2019, 146, 78–86. [Google Scholar] [CrossRef]
- Islam, M.; Kitamura, Y.; Yamano, Y.; Kitamura, M. Effect of vacuum spray drying on the physicochemical properties, water sorption and glass transition phenomenon of orange juice powder. J. Food Eng. 2016, 169, 131–140. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Parize, A.L.; Stulzer, H.K.; Laranjeira, M.C.M.; Brighente, I.M.d.C.; Souza, T.C. Evaluation of chitosan microparticles containing curcumin and crosslinked with sodium tripolyphosphate produced by spray drying. Química Nova 2012, 35, 1127–1132. [Google Scholar] [CrossRef] [Green Version]
- Khatun, B.; Banik, N.; Hussain, A.; Ramteke, A.; Maji, T. Genipin crosslinked curcumin loaded chitosan/montmorillonite K-10 (MMT) nanoparticles for controlled drug delivery applications. J. Microencapsul. 2018, 35, 439–453. [Google Scholar] [CrossRef]
- Neves, M.I.L.; Desobry-Banon, S.; Perrone, I.T.; Desobry, S.; Petit, J. Encapsulation of curcumin in milk powders by spray-drying: Physicochemistry, rehydration properties, and stability during storage. Powder Technol. 2019, 345, 601–607. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Núñez-Ramírez, D.M.; Calderas, F.; Bernad-Bernad, M.; Gracia-Mora, J.; Rodríguez-Ramírez, J.; González-Laredo, R.; Gallegos-Infante, J.; Manero, O. Curcumin encapsulation by spray drying using Aloe vera mucilage as encapsulating agent. J. Food Process Eng. 2019, 42, e12972. [Google Scholar] [CrossRef]
- Guo, J.; Li, P.; Kong, L.; Xu, B. Microencapsulation of curcumin by spray drying and freeze drying. LWT 2020, 132, 109892. [Google Scholar] [CrossRef]
- Ponrasu, T.; Yang, R.-F.; Chou, T.-H.; Wu, J.-J.; Cheng, Y.-S. Core-Shell Encapsulation of Lipophilic Substance in Jelly Fig (Ficus awkeotsang Makino) Polysaccharides Using an Inexpensive Acrylic-Based Millifluidic Device. Appl. Biochem. Biotechnol. 2020, 191, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Masuelli, M.; Blumenberg, M. Pectins: Extraction, Purification, Characterization and Applications; BoD–Books on Demand: Hamburg, Germany, 2020. [Google Scholar]
- Ponrasu, T.; Gu, J.-S.; Wu, J.-J.; Cheng, Y.-S. Evaluation of jelly fig polysaccharide as a shell composite ingredient of colon-specific drug delivery. J. Drug Deliv. Sci. Technol. 2021, 61, 101679. [Google Scholar] [CrossRef]
- Lai, Y.-J.; Chen, J.-N.; Wu, J.S.-B. Antibacterial activity and antioxidant properties of water extract from the residue of jelly fig (Ficus awkeotsang Makino) achenes. J. Food Drug Anal. 2008, 16, 7. [Google Scholar]
- Quintero Quiroz, J.; Velazquez, V.; Corrales-Garcia, L.L.; Torres, J.D.; Delgado, E.; Ciro, G.; Rojas, J. Use of Plant Proteins as Microencapsulating Agents of Bioactive Compounds Extracted from Annatto Seeds (Bixa orellana L.). Antioxidants 2020, 9, 310. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Hubinger, M.D. Hydrophilic food compounds encapsulation by ionic gelation. Curr. Opin. Food Sci. 2017, 15, 50–55. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Yakou, S.; Takayama, K. Study on jelly fig extract as a potential hydrophilic matrix for controlled drug delivery. Int. J. Pharm. 2004, 287, 39–46. [Google Scholar] [CrossRef]
- Torres, D.; Seijo, B.; García-Encina, G.; Alonso, M.; Vila-Jato, J.L. Microencapsulation of ion-exchange resins by interfacial nylon polymerization. Int. J. Pharm. 1990, 59, 9–17. [Google Scholar] [CrossRef]
- Schuck, P.; Jeantet, R.; Dolivet, A. Analytical Methods for Food and Dairy Powders; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Liu, W.; Chen, X.D.; Cheng, Z.; Selomulya, C. On enhancing the solubility of curcumin by microencapsulation in whey protein isolate via spray drying. J. Food Eng. 2016, 169, 189–195. [Google Scholar] [CrossRef]
- Govindaraju, R.; Karki, R.; Chandrashekarappa, J.; Santhanam, M.; Shankar, A.K.; Joshi, H.K.; Divakar, G. Enhanced water dispersibility of curcumin encapsulated in alginate-polysorbate 80 nano particles and bioavailability in healthy human volunteers. Pharm. Nanotechnol. 2019, 7, 39–56. [Google Scholar] [CrossRef]
- Subudhi, M.B.; Jain, A.; Jain, A.; Hurkat, P.; Shilpi, S.; Gulbake, A.; Jain, S.K. Eudragit S100 coated citrus pectin nanoparticles for colon targeting of 5-fluorouracil. Materials 2015, 8, 832–849. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S. Encapsulation of Omega-3 Fatty Acids by Premix Membrane Emulsification; Universitat Rovira i Virgili: Tarragona, Spain, 2014. [Google Scholar]
- Garcia, L.C.; Tonon, R.V.; Hubinger, M.D. Effect of homogenization pressure and oil load on the emulsion properties and the oil retention of microencapsulated basil essential oil (Ocimum basilicum L.). Dry. Technol. 2012, 30, 1413–1421. [Google Scholar] [CrossRef]
- Böger, B.R.; Georgetti, S.R.; Kurozawa, L.E. Technology. Microencapsulation of grape seed oil by spray drying. Food Sci. Technol. 2018, 38, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, B.F.; Kermasha, S.; Alli, I.; Mulligan, C.N. Encapsulation in the food industry: A review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar]
- Li, X.; Anton, N.; Arpagaus, C.; Belleteix, F.; Vandamme, T.F. Nanoparticles by spray drying using innovative new technology: The Büchi Nano Spray Dryer B-90. J. Control. Release 2010, 147, 304–310. [Google Scholar] [CrossRef]
- Mis Solval, K.E. Spray Drying Technology for the Production and Processing of Microencapsulated Omega-3 Fish Oil with Egg Powder. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2011. [Google Scholar]
- da Rosa, J.R.; Weis, G.C.C.; Moro, K.I.B.; Robalo, S.S.; Assmann, C.E.; da Silva, L.P.; Muller, E.I.; da Silva, C.d.B.; de Menezes, C.R.; da Rosa, C.S. Effect of wall materials and storage temperature on anthocyanin stability of microencapsulated blueberry extract. LWT 2021, 142, 111027. [Google Scholar] [CrossRef]
- Pérez-Alonso, C.; Báez-González, J.; Beristain, C.; Vernon-Carter, E.; Vizcarra-Mendoza, M. Estimation of the activation energy of carbohydrate polymers blends as selection criteria for their use as wall material for spray-dried microcapsules. Carbohydr. Polym. 2003, 53, 197–203. [Google Scholar] [CrossRef]
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef]
- El Ghannam, M.; El Nemr, T.; Hassan, A.; Dyab, N. Encapsulation efficiency, microstructure and oxidation stability of fish oil encapsulated powder made by using whey protein concentrate. Alex. Sci. Exch. J. 2015, 36, 236–248. [Google Scholar]
- Akyüz, L.; Duman, F. Encapsulation of flurbiprofen by chitosan using a spray-drying method with in vitro drug releasing and molecular docking. Turk. J. Pharm. Sci. 2017, 14, 34. [Google Scholar] [CrossRef]
- Jadupati, M.; Tanmay, D.; Souvik, G. Microencapsulation: An indispensable technology for drug delivery system. Int. Res. J. Pharm. 2012, 3, 8–13. [Google Scholar]
- Marathe, R. Development of Controlled Release Antimicrobial Films from Low Methoxyl Pectin; Rutgers University-Graduate School-New Brunswick: New Brunswick, NJ, USA, 2008. [Google Scholar]
- Rosenberg, M.; Rosenberg, Y.; Frenkel, L. Microencapsulation of model oil in wall matrices consisting of SPI and maltodextrins. AIMS Agric. Food 2016, 1, 33–51. [Google Scholar] [CrossRef]
- Krishnan, S.; Bhosale, R.; Singhal, R.S. Microencapsulation of cardamom oleoresin: Evaluation of blends of gum arabic, maltodextrin and a modified starch as wall materials. Carbohydr. Polym. 2005, 61, 95–102. [Google Scholar] [CrossRef]
- Ganesh, S.; Kumar, D.; Kumar, B.S.; Abhilash, R.; Bharadwaj, P.S.; Raj, K.; Mohammed, I.; Pravalika, T. Controlled release formulation and evaluation of idarubicin microsphere using biodegradable hydrophilic and hydrophobic polymer mixtures. Asian J. Pharm. Clin. Res. 2010, 3, 179–182. [Google Scholar]
- Ismail, E.; Sabry, D.; Mahdy, H.; Khalil, M. Synthesis and Characterization of some Ternary Metal Complexes of Curcumin with 1, 10-phenanthroline and their Anticancer Applications. J. Sci. Res. 2014, 6, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Villanova, J.; Ayres, E.; Oréfice, R. Design, characterization and preliminary in vitro evaluation of a mucoadhesive polymer based on modified pectin and acrylic monomers with potential use as a pharmaceutical excipient. Carbohydr. Polym. 2015, 121, 372–381. [Google Scholar] [CrossRef]
- Ren, Y.; Jiang, L.; Yang, S.; Gao, S.; Yu, H.; Hu, J.; Hu, D.; Mao, W.; Peng, H.; Zhou, Y. Design and preparation of a novel colon-targeted tablet of hydrocortisone. Braz. J. Pharm. Sci. 2016, 52, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Cuma, D.F. Microencapsulation Using Alginate Systems: Spray-Coagulation Versus Superhydrophobic Surfaces Approach. Master’s Thesis, Instituto Politécnico de Bragança, Bragança, Portugal, 2018. [Google Scholar]
- Ramasamy, T.; Kandhasami, U.D.S.; Ruttala, H.; Shanmugam, S. Formulation and evaluation of xanthan gum based aceclofenac tablets for colon targeted drug delivery. Braz. J. Pharm. Sci. 2011, 47, 299–311. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Nisar, T.; Liang, D.; Hou, Y.; Sun, L.; Guo, Y. Low methoxyl pectin gelation under alkaline conditions and its rheological properties: Using NaOH as a pH regulator. Food Hydrocoll. 2018, 79, 560–571. [Google Scholar] [CrossRef]
- Sánchez-Silva, L.; Rodríguez, J.F.; Carmona, M.; Romero, A.; Sánchez, P. Thermal and morphological stability of polystyrene microcapsules containing phase-change materials. J. Appl. Polym. Sci. 2011, 120, 291–297. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Silva, H.D.; Soliva-Fortuny, R.; Martín-Belloso, O.; Vicente, A.A. Formation, stability and antioxidant activity of food-grade multilayer emulsions containing resveratrol. Food Hydrocoll. 2017, 71, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Okuro, P.K.; de Matos Junior, F.E.; Favaro-Trindade, C.S. Technological challenges for spray chilling encapsulation of functional food ingredients. Food Technol. Biotechnol. 2013, 51, 171. [Google Scholar]










| Formulation | Dried Powder, g | Yield, w/w% | Moisture Content, w/w% |
|---|---|---|---|
| C-0.75JFMs 80 °C | 4.63 ± 0.02 | 64.02 ± 0.17 | 6.64 ± 0.82 |
| C-0.75JFMs 90 °C | 5.07 ± 0.15 | 70.02 ± 1.96 | 5.51 ± 0.89 |
| C-1.5JFMs 80 °C | 5.53 ± 0.06 | 63.09 ± 1.00 | 6.44 ± 0.94 |
| C-1.5JFMs 90 °C | 5.62 ± 0.02 | 64.09 ± 0.30 | 6.36 ± 1.80 |
| C-2.25JFMs 80 °C | 5.72 ± 1.71 | 56.04 ± 6.78 | 6.16 ± 2.53 |
| C-2.25JFMs 90 °C | 6.32 ± 0.93 | 62.00 ± 9.10 | 3.84 ± 2.93 |
| Formulation | Total Curcumin, µg/mg | Free Curcumin, µg/mg | LE, % | EE, % |
|---|---|---|---|---|
| C-0.75FMs 80 °C | 58.14 ± 0.58 | 18.03 ± 0.69 | 4.01 ± 0.07 | 68.95 ± 1.14 |
| C-0.75FMs 90 °C | 64.07 ± 1.36 | 9.80 ± 0.33 | 5.45 ± 0.14 | 84.77 ± 0.98 |
| C-1.5FMs 80 °C | 53.44 ± 1.29 | 10.71 ± 0.36 | 4.27 ± 0.10 | 79.95 ± 0.39 |
| C-1.5FMs 90 °C | 55.86 ± 1.80 | 6.59 ± 0.12 | 4.90 ± 0.17 | 88.19 ± 0.22 |
| C-2.25FMs 80 °C | 48.23 ± 1.29 | 4.24 ± 0.38 | 4.40 ± 0.16 | 91.07 ± 1.00 |
| C-2.25FMs 90 °C | 50.98 ± 1.80 | 4.32 ± 0.30 | 4.69 ± 0.19 | 91.56 ± 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartini, N.; Ponrasu, T.; Wu, J.-J.; Sriariyanun, M.; Cheng, Y.-S. Microencapsulation of Curcumin in Crosslinked Jelly Fig Pectin Using Vacuum Spray Drying Technique for Effective Drug Delivery. Polymers 2021, 13, 2583. https://doi.org/10.3390/polym13162583
Hartini N, Ponrasu T, Wu J-J, Sriariyanun M, Cheng Y-S. Microencapsulation of Curcumin in Crosslinked Jelly Fig Pectin Using Vacuum Spray Drying Technique for Effective Drug Delivery. Polymers. 2021; 13(16):2583. https://doi.org/10.3390/polym13162583
Chicago/Turabian StyleHartini, Nina, Thangavel Ponrasu, Jia-Jiuan Wu, Malinee Sriariyanun, and Yu-Shen Cheng. 2021. "Microencapsulation of Curcumin in Crosslinked Jelly Fig Pectin Using Vacuum Spray Drying Technique for Effective Drug Delivery" Polymers 13, no. 16: 2583. https://doi.org/10.3390/polym13162583
APA StyleHartini, N., Ponrasu, T., Wu, J. -J., Sriariyanun, M., & Cheng, Y. -S. (2021). Microencapsulation of Curcumin in Crosslinked Jelly Fig Pectin Using Vacuum Spray Drying Technique for Effective Drug Delivery. Polymers, 13(16), 2583. https://doi.org/10.3390/polym13162583