Polymer-Based Nanofiber–Nanoparticle Hybrids and Their Medical Applications
Abstract
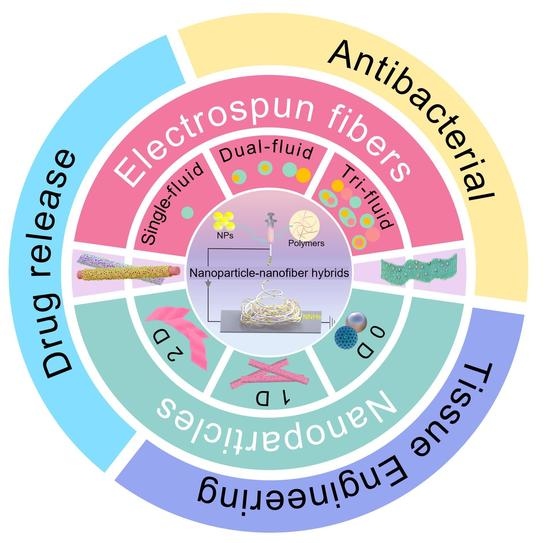
:1. Introduction
2. The Methods for Creating Polymer-Based Nanofiber-Nanoparticle Hybrids (NNHs)
2.1. Overlapping of Electrospinning and Other Techniques
2.2. Encapsulating Nanoparticles in the Working Fluids
2.3. Formation of NNHs in the Single-Step Process
2.4. The Nanoparticles from Nanofibers through Molecular Self-Assembly
2.4.1. In Situ Synthesis
2.4.2. Calcination
2.4.3. Hydrothermal-Assistance
3. The Biomedical Applications of Nanofiber-Nanoparticle Hybrids (NNHs)
3.1. Drug Delivery
3.1.1. One-Drug Biphasic
3.1.2. Dual-Drug Biphasic Approach
3.1.3. Smart Response Drug Delivery
3.2. Antibacterial
3.3. Tissue Engineering
4. The Present Challenges of NNHs in Medical Applications
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koh, H.K.; Geller, A.C.; VanderWeele, T.J. Deaths From COVID-19. J. Am. Med. Assoc. 2021, 325, 133–134. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S.; Merad, M.; Swaminathan, S.; Hur, S.; Topol, E.; Fitzgerald, K.; Reis e Sousa, C.; Corbett, K.S.; Bauer, S. From MRNA Sensing to Vaccines. Immunity 2021, 54, 2676–2680. [Google Scholar] [CrossRef] [PubMed]
- Extance, A. MRNA Vaccines: Hope beneath the Hype. Br. Med. J. 2021, 375, n2744. [Google Scholar] [CrossRef]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the MRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef]
- Ali, K.; Berman, G.; Zhou, H.; Deng, W.; Faughnan, V.; Coronado-Voges, M.; Ding, B.; Dooley, J.; Girard, B.; Hillebrand, W.; et al. Evaluation of MRNA-1273 SARS-CoV-2 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 2241–2251. [Google Scholar] [CrossRef]
- Becker, M.L.; Burdick, J.A. Introduction: Polymeric Biomaterials. Chem. Rev. 2021, 121, 10789–10791. [Google Scholar] [CrossRef]
- Safian, M.T.; Umar, K.; Parveen, T.; Yaqoob, A.A.; Ibrahim, M.N.M. Chapter Eight-Biomedical applications of smart polymer composites. In Smart Polymer Nanocomposites: Biomedical and Environmental Applications; Elsevier Inc.: Cambridge, MA, USA, 2021; pp. 183–204. [Google Scholar]
- Chakraborty, P.; Oved, H.; Bychenko, D.; Yao, Y.; Tang, Y.; Zilberzwige-Tal, S.; Wei, G.; Dvir, T.; Gazit, E. Nanoengineered Peptide-Based Antimicrobial Conductive Supramolecular Biomaterial for Cardiac Tissue Engineering. Adv. Mater. 2021, 33, 2008715. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.G.; Wang, L.; Li, X.; Williams, G.R.R. Energy-saving electrospinning with a concentric Teflon-core rod spinneret to create medicated nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Safian, M.T.; Rashid, M.; Parveen, T.; Umar, K.; Ibrahim, M.N.M. Chapter One-Introduction of Smart Polymer Nanocomposites. In Smart Polymer Nanocomposites: Biomedical and Environmental Applications; Elsevier Inc.: Cambridge, MA, USA, 2021; pp. 1–25. [Google Scholar]
- Jung, K.; Corrigan, N.; Wong, E.H.H.; Boyer, C. Bioactive Synthetic Polymers. Adv. Mater. 2021, 34, 2105063. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A. Collagen Blended with Natural Polymers: Recent Advances and Trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive Potential of Natural Biomaterials: Identification, Retention and Assessment of Biological Properties. Signal Transduc. Target. Ther. 2021, 6, 1–28. [Google Scholar] [CrossRef]
- Rosén, T.; Hsiao, B.S.; Söderberg, L.D. Elucidating the Opportunities and Challenges for Nanocellulose Spinning. Adv. Mater. 2021, 33, 2001238. [Google Scholar] [CrossRef]
- Taokaew, S.T.; Chiaoprakobkij, N.; Siripong, P.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Multifunctional Cellulosic Nanofiber Film with Enhanced Antimicrobial and Anticancer Properties by Incorporation of Ethanolic Extract of Garcinia Mangostana Peel. Mater. Sci. Eng. C 2021, 120, 111783. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Q.; Li, H.; Wei, Q.; Zhao, X.; Chen, F. Elastin-like Polypeptide Modified Silk Fibroin Porous Scaffold Promotes Osteochondral Repair. Bioact. Mater. 2021, 6, 589–601. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Nateghi, S.S.; Gazani, M.M.; Dehghani, Z.; Mohammadzadeh, F. A Review on Advances in the Applications of Spider Silk in Biomedical Issues. Int. J. Biol. Macromol. 2021, 192, 258–271. [Google Scholar] [CrossRef]
- Dziadek, M.; Dziadek, K.; Checinska, K.; Zagrajczuk, B.; Golda-Cepa, M.; Brzychczy-Wloch, M.; Menaszek, E.; Kopec, A.; Cholewa-Kowalska, K. PCL and PCL/Bioactive Glass Biomaterials as Carriers for Biologically Active Polyphenolic Compounds: Comprehensive Physicochemical and Biological Evaluation. Bioact. Mater. 2021, 6, 1811–1826. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nourbakhsh, M.S. Electrospun Polycaprolactone Scaffolds for Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 527–539. [Google Scholar] [CrossRef]
- Zavan, B.; Gardin, C.; Guarino, V.; Rocca, T.; Cruz Maya, I.; Zanotti, F.; Ferroni, L.; Brunello, G.; Chachques, J.-C.; Ambrosio, L.; et al. Electrospun PCL-Based Vascular Grafts: In Vitro Tests. Nanomaterials 2021, 11, 751. [Google Scholar] [CrossRef]
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.-G. Electrospun Structural Hybrids of Acyclovir-Polyacrylonitrile at Acyclovir for Modifying Drug Release. Polymers 2021, 13, 4286. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.; Qin, Y.; Li, C. Functional Electrospun Nanocomposites for Efficient Oxygen Reduction Reaction. Chem. Res. Chin. Univ. 2021, 37, 379–393. [Google Scholar] [CrossRef]
- Ning, T.; Zhou, Y.; Xu, H.; Guo, S.; Wang, K.; Yu, D.-G. Orodispersible Membranes from a Modified Coaxial Electrospinning for Fast Dissolution of Diclofenac Sodium. Membranes 2021, 11, 802. [Google Scholar] [CrossRef]
- Xu, H.; Xu, X.; Li, S.; Song, W.-L.; Yu, D.-G.; Annie Bligh, S.W. The Effect of Drug Heterogeneous Distributions within Core-Sheath Nanostructures on Its Sustained Release Profiles. Biomolecules 2021, 11, 1330. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Wu, M.; Yu, D.-G. Nanofibers-Based Food Packaging. ES Food Agrofor. 2022, 2, 1–23. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.-G. Electrospun Medicated Nanofibers for Wound Healing: Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, K.; Yu, D.G.; Zheng, X.; Huang, C. Advances in Biosensing and Environmental Monitoring Based on Electrospun Nanofibers. Adv. Fiber Mater. 2022, 9. [Google Scholar] [CrossRef]
- Zhao, K.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Electrospun Functional Nanofiber Membrane for Antibiotic Removal in Water: Review. Polymers 2021, 13, 226. [Google Scholar] [CrossRef]
- Shepa, I.; Mudra, E.; Dusza, J. Electrospinning through the Prism of Time. Mater. Today Chem. 2021, 21, 100543. [Google Scholar] [CrossRef]
- Madruga, L.Y.C.; Kipper, M.J. Expanding the Repertoire of Electrospinning: New and Emerging Biopolymers, Techniques, and Applications. Adv. Heal. Mater. 2021, 2101979. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, L.; Wang, X.; Shao, Z.; Kong, B. Electrospinning Super-Assembly of Ultrathin Fibers from Single- to Multi-Taylor Cone Sites. Appl. Mater. Today 2021, 23, 101272. [Google Scholar] [CrossRef]
- Wang, X.-X.; Yu, G.-F.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Conductive Polymer Ultrafine Fibers via Electrospinning: Preparation, Physical Properties and Applications. Prog. Mater. Sci. 2021, 115, 100704. [Google Scholar] [CrossRef]
- Filip, P.; Zelenkova, J.; Peer, P. Electrospinning of a Copolymer PVDF-Co-HFP Solved in DMF/Acetone: Explicit Relations among Viscosity, Polymer Concentration, DMF/Acetone Ratio and Mean Nanofiber Diameter. Polymers 2021, 13, 3418. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kong, L.; Ziegler, G.R. Electrospinning of Octenylsuccinylated Starch-Pullulan Nanofibers from Aqueous Dispersions. Carbohydr. Polym. 2021, 258, 116933. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, S.; Li, Y.; Wang, Z.; Tang, J. Effect of Chemical Structure and Molecular Weight on the Properties of Lignin-Based Ultrafine Carbon Fibers. Int. J. Biol. Macromol. 2021, 187, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Tian, X.; Zheng, L.; Jin, X.; Zhang, Q.; Xu, S.; Liu, P.; Yang, N.; Bai, H.; Wang, H. Yeast Encapsulation in Nanofiber via Electrospinning: Shape Transformation, Cell Activity and Immobilized Efficiency. Mater. Sci. Eng. C 2021, 120, 111747. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xu, C.; Yu, H.; Feng, Z.; Yu, W.; Gu, L.; Liu, Z.; Chen, L.; Jiang, Z.; Hou, J. Electro-Encapsulation of Probiotics in Gum Arabic-Pullulan Blend Nanofibres Using Electrospinning Technology. Food Hydrocoll. 2021, 111, 106381. [Google Scholar] [CrossRef]
- Silva, P.M.; Prieto, C.; Lagarón, J.M.; Pastrana, L.M.; Coimbra, M.A.; Vicente, A.A.; Cerqueira, M.A. Food-Grade Hydroxypropyl Methylcellulose-Based Formulations for Electrohydrodynamic Processing: Part I–Role of Solution Parameters on Fibre and Particle Production. Food Hydrocoll. 2021, 118, 106761. [Google Scholar] [CrossRef]
- Topuz, F.; Abdulhamid, M.A.; Holtzl, T.; Szekely, G. Nanofiber Engineering of Microporous Polyimides through Electrospinning: Influence of Electrospinning Parameters and Salt Addition. Mater. Des. 2021, 198, 109280. [Google Scholar] [CrossRef]
- Joy, N.; Anuraj, R.; Viravalli, A.; Dixit, H.N.; Samavedi, S. Coupling between Voltage and Tip-to-Collector Distance in Polymer Electrospinning: Insights from Analysis of Regimes, Transitions and Cone/Jet Features. Chem. Eng. Sci. 2021, 230, 116200. [Google Scholar] [CrossRef]
- Lei, L.; Chen, S.; Nachtigal, C.J.; Moy, T.F.; Yong, X.; Singer, J.P. Homogeneous Gelation Leads to Nanowire Forests in the Transition between Electrospray and Electrospinning. Mater. Horiz. 2020, 7, 2643–2650. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Jiang, L.; Zhao, L.; Wang, J.; Liu, F.; Wang, C.; Yan, X.; Sun, P.; Wang, L.; et al. Mixed Potential Type YSZ-Based NO2 Sensors with Efficient Three-Dimensional Three-Phase Boundary Processed by Electrospinning. Sens. Actuators B Chem. 2022, 354, 131219. [Google Scholar] [CrossRef]
- Wang, C.; Zuo, Q.; Wang, L.; Long, B.; Salleh, K.M.; Anuar, N.I.S.; Zakaria, S. Diameter Optimization of Polyvinyl Alcohol/Sodium Alginate Fiber Membranes Using Response Surface Methodology. Mater. Chem. Phys. 2021, 271, 124969. [Google Scholar] [CrossRef]
- Kopp, A.; Smeets, R.; Gosau, M.; Kröger, N.; Fuest, S.; Köpf, M.; Kruse, M.; Krieger, J.; Rutkowski, R.; Henningsen, A.; et al. Effect of Process Parameters on Additive-Free Electrospinning of Regenerated Silk Fibroin Nonwovens. Bioact. Mater. 2020, 5, 241–252. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Yu, H.; Jia, Y.; Wang, X.; Ding, B. Self-Assembly of Polyethylene Oxide and Its Composite Nanofibrous Membranes with Cellular Network Structure. Compos. Commun. 2021, 27, 100759. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Zhang, H.; Zhao, C.; Sun, L.; Zhao, Y. Emerging Functional Biomaterials as Medical Patches. ACS Nano 2021, 15, 5977–6007. [Google Scholar] [CrossRef]
- Huo, P.; Han, X.; Zhang, W.; Zhang, J.; Kumar, P.; Liu, B. Electrospun Nanofibers of Polycaprolactone/Collagen as a Sustained-Release Drug Delivery System for Artemisinin. Pharmaceutics 2021, 13, 1228. [Google Scholar] [CrossRef]
- Chen, K.; Pan, H.; Yan, Z.; Li, Y.; Ji, D.; Yun, K.; Su, Y.; Liu, D.; Pan, W. A Novel Alginate/Gelatin Sponge Combined with Curcumin-Loaded Electrospun Fibers for Postoperative Rapid Hemostasis and Prevention of Tumor Recurrence. Int. J. Biol. Macromol. 2021, 182, 1339–1350. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Ouyang, J.; Zhang, L.; Xue, J.; Zhang, H.; Tao, W. Electroactive Electrospun Nanofibers for Tissue Engineering. Nano Today 2021, 39, 101196. [Google Scholar] [CrossRef]
- Zhu, M.; Tan, J.; Liu, L.; Tian, J.; Li, L.; Luo, B.; Zhou, C.; Lu, L. Construction of Biomimetic Artificial Intervertebral Disc Scaffold via 3D Printing and Electrospinning. Mater. Sci. Eng. C 2021, 128, 112310. [Google Scholar] [CrossRef]
- Rickel, A.P.; Deng, X.; Engebretson, D.; Hong, Z. Electrospun Nanofiber Scaffold for Vascular Tissue Engineering. Mater. Sci. Eng. C 2021, 129, 112373. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Shao, C.; Lin, X.; Yang, Y.; Chen, N.; Wang, Y.; Qu, L. Moisture Power in Natural Polymeric Silk Fibroin Flexible Membrane Triggers Efficient Antibacterial Activity of Silver Nanoparticles. Nano Energy 2021, 90, 106529. [Google Scholar] [CrossRef]
- Ho Na, J.; Chan Kang, Y.; Park, S.-K. Electrospun MOF-Based ZnSe Nanocrystals Confined in N-Doped Mesoporous Carbon Fibers as Anode Materials for Potassium Ion Batteries with Long-Term Cycling Stability. Chem. Eng. J. 2021, 425, 131651. [Google Scholar] [CrossRef]
- Zhao, K.; Lu, Z.-H.; Zhao, P.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Modified Tri–Axial Electrospun Functional Core–Shell Nanofibrous Membranes for Natural Photodegradation of Antibiotics. Chem. Eng. J. 2021, 425, 131455. [Google Scholar] [CrossRef]
- Huang, G.-Y.; Chang, W.-J.; Lu, T.-W.; Tsai, I.-L.; Wu, S.-J.; Ho, M.-H.; Mi, F.-L. Electrospun CuS Nanoparticles/Chitosan Nanofiber Composites for Visible and near-Infrared Light-Driven Catalytic Degradation of Antibiotic Pollutants. Chem. Eng. J. 2022, 431, 134059. [Google Scholar] [CrossRef]
- Cui, Z.; Shen, S.; Yu, J.; Si, J.; Cai, D.; Wang, Q. Electrospun Carbon Nanofibers Functionalized with NiCo2S4 Nanoparticles as Lightweight, Flexible and Binder-Free Cathode for Aqueous Ni-Zn Batteries. Chem. Eng. J. 2021, 426, 130068. [Google Scholar] [CrossRef]
- Sharma, D.; Satapathy, B.K. Polymer Substrate-Based Transition Metal Modified Electrospun Nanofibrous Materials: Current Trends in Functional Applications and Challenges. Polym. Rev. 2021, 61, 1–46. [Google Scholar] [CrossRef]
- Jena, S.K.; Sadasivam, R.; Packirisamy, G.; Saravanan, P. Nanoremediation: Sunlight Mediated Dye Degradation Using Electrospun PAN/CuO–ZnO Nanofibrous Composites. Environ. Pollut. 2021, 280, 116964. [Google Scholar] [CrossRef]
- Ding, C.; Breunig, M.; Timm, J.; Marschall, R.; Senker, J.; Agarwal, S. Flexible, Mechanically Stable, Porous Self-Standing Microfiber Network Membranes of Covalent Organic Frameworks: Preparation Method and Characterization. Adv. Funct. Mater. 2021, 31, 2106507. [Google Scholar] [CrossRef]
- Neibolts, N.; Platnieks, O.; Gaidukovs, S.; Barkane, A.; Thakur, V.K.; Filipova, I.; Mihai, G.; Zelca, Z.; Yamaguchi, K.; Enachescu, M. Needle-Free Electrospinning of Nanofibrillated Cellulose and Graphene Nanoplatelets Based Sustainable Poly (Butylene Succinate) Nanofibers. Mater. Today Chem. 2020, 17, 100301. [Google Scholar] [CrossRef]
- Ren, S.; Zhou, Y.; Zheng, K.; Xu, X.; Yang, J.; Wang, X.; Miao, L.; Wei, H.; Xu, Y. Cerium Oxide Nanoparticles Loaded Nanofibrous Membranes Promote Bone Regeneration for Periodontal Tissue Engineering. Bioact. Mater. 2022, 7, 242–253. [Google Scholar] [CrossRef]
- Rasekh, A.; Raisi, A. Electrospun Nanofibrous Polyether-Block-Amide Membrane Containing Silica Nanoparticles for Water Desalination by Vacuum Membrane Distillation. Sep. Purif. Technol. 2021, 275, 119149. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, S.; Zou, B.; Li, G.; Yang, S.; Zhao, Y.; Lian, J.; Li, H.; Ji, H. Electrospun CoSe@NC Nanofiber Membrane as an Effective Polysulfides Adsorption-Catalysis Interlayer for Li-S Batteries. Chem. Eng. J. 2022, 430, 131911. [Google Scholar] [CrossRef]
- Fang, H.; Wang, C.; Li, D.; Zhou, S.; Du, Y.; Zhang, H.; Hang, C.; Tian, Y.; Suga, T. Fabrication of Ag@Ag2O-MnOx Composite Nanowires for High-Efficient Room-Temperature Removal of Formaldehyde. J. Mater. Sci. Technol. 2021, 91, 5–16. [Google Scholar] [CrossRef]
- Park, K.; Kang, S.; Park, J.; Hwang, J. Fabrication of Silver Nanowire Coated Fibrous Air Filter Medium via a Two-Step Process of Electrospinning and Electrospray for Anti-Bioaerosol Treatment. J. Hazard. Mater. 2021, 411, 125043. [Google Scholar] [CrossRef]
- Enculescu, M.; Costas, A.; Evanghelidis, A.; Enculescu, I. Fabrication of ZnO and TiO2 Nanotubes via Flexible Electro-Spun Nanofibers for Photocatalytic Applications. Nanomaterials 2021, 11, 1305. [Google Scholar] [CrossRef]
- Wang, K.; Wang, P.; Wang, M.; Yu, D.-G.; Wan, F.; Bligh, S.W.A. Comparative Study of Electrospun Crystal-Based and Composite-Based Drug Nano Depots. Mater. Sci. Eng. C 2020, 113, 110988. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.-G.; Li, S.; Zhu, J.; Chen, Z. Electrospun Tri-Layer Nanodepots for Sustained Release of Acyclovir. J. Alloy. Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Zheng, G.; Peng, H.; Jiang, J.; Kang, G.; Liu, J.; Zheng, J.; Liu, Y. Surface Functionalization of PEO Nanofibers Using a TiO2 Suspension as Sheath Fluid in a Modified Coaxial Electrospinning Process. Chem. Res. Chin. Univ. 2021, 37, 571–577. [Google Scholar] [CrossRef]
- Yang, C.-H.; Hsiao, Y.-C.; Lin, L.-Y. Novel in Situ Synthesis of Freestanding Carbonized ZIF67/Polymer Nanofiber Electrodes for Supercapacitors via Electrospinning and Pyrolysis Techniques. ACS Appl. Mater. Interfaces 2021, 13, 41637–41648. [Google Scholar] [CrossRef]
- Pant, B.; Prasad Ojha, G.; Acharya, J.; Park, M. Ag3PO4-TiO2-Carbon Nanofiber Composite: An Efficient Visible-Light Photocatalyst Obtained from Eelectrospinning and Hydrothermal Methods. Sep. Purif. Technol. 2021, 276, 119400. [Google Scholar] [CrossRef]
- Li, R.; Ke, H.; Shi, C.; Long, Z.; Dai, Z.; Qiao, H.; Wang, K. Mesoporous RGO/NiCo2O4@carbon Composite Nanofibers Derived from Metal-Organic Framework Compounds for Lithium Storage. Chem. Eng. J. 2021, 415, 128874. [Google Scholar] [CrossRef]
- Alturki, A.M. Rationally Design of Electrospun Polysaccharides Polymeric Nanofiber Webs by Various Tools for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2021, 184, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun Polymer Biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Vitali, R.; Guillot, G.; Rinaldi, A. Sputter-Deposited Ag Nanoparticles on Electrospun PCL Scaffolds: Morphology, Wettability and Antibacterial Activity. Coatings 2021, 11, 345. [Google Scholar] [CrossRef]
- Liu, Z.; Jia, L.; Yan, Z.; Bai, L. Plasma-Treated Electrospun Nanofibers as a Template for the Electrostatic Assembly of Silver Nanoparticles. N. J. Chem. 2018, 42, 11185–11191. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Rostamabadi, M.M.; Assadpour, E.; Jafari, S.M. Electrospraying as a Novel Process for the Synthesis of Particles/Nanoparticles Loaded with Poorly Water-Soluble Bioactive Molecules. Adv. Colloid Interface Sci. 2021, 290, 102384. [Google Scholar] [CrossRef]
- Fahimirad, S.; Abtahi, H.; Satei, P.; Ghaznavi-Rad, E.; Moslehi, M.; Ganji, A. Wound Healing Performance of PCL/Chitosan Based Electrospun Nanofiber Electrosprayed with Curcumin Loaded Chitosan Nanoparticles. Carbohydr. Polym. 2021, 259, 117640. [Google Scholar] [CrossRef] [PubMed]
- Karagoz, S.; Kiremitler, N.B.; Sarp, G.; Pekdemir, S.; Salem, S.; Goksu, A.G.; Onses, M.S.; Sozdutmaz, I.; Sahmetlioglu, E.; Ozkara, E.S.; et al. Antibacterial, Antiviral, and Self-Cleaning Mats with Sensing Capabilities Based on Electrospun Nanofibers Decorated with ZnO Nanorods and Ag Nanoparticles for Protective Clothing Applications. ACS Appl. Mater. Interfaces 2021, 13, 5678–5690. [Google Scholar] [CrossRef]
- Lopresti, F.; Pavia, F.C.; Ceraulo, M.; Capuana, E.; Brucato, V.; Ghersi, G.; Botta, L.; La Carrubba, V. Physical and Biological Properties of Electrospun Poly(d,l-Lactide)/Nanoclay and Poly(d,l-Lactide)/Nanosilica Nanofibrous Scaffold for Bone Tissue Engineering. J. Biomed. Mater. Res. Part A 2021, 109, 2120–2136. [Google Scholar] [CrossRef]
- Abdelaziz, D.; Hefnawy, A.; Al-Wakeel, E.; El-Fallal, A.; El-Sherbiny, I.M. New Biodegradable Nanoparticles-in-Nanofibers Based Membranes for Guided Periodontal Tissue and Bone Regeneration with Enhanced Antibacterial Activity. J. Adv. Res. 2021, 28, 51–62. [Google Scholar] [CrossRef]
- Navarro Oliva, F.S.; Picart, L.; Leon-Valdivieso, C.Y.; Benalla, A.; Lenglet, L.; Ospina, A.; Jestin, J.; Bedoui, F. Coaxial Electrospinning Process toward Optimal Nanoparticle Dispersion in Polymeric Matrix. Polym. Compos. 2021, 42, 1565–1573. [Google Scholar] [CrossRef]
- Radacsi, N.; Campos, F.D.; Chisholm, C.R.I.; Giapis, K.P. Spontaneous Formation of Nanoparticles on Electrospun Nanofibres. Nat. Commun. 2018, 9, 4740. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.G.; Lv, H. Preface-striding into Nano Drug Delivery. Curr. Drug Deliv. 2022, 19, 1–3. [Google Scholar]
- Ding, Y.; Dou, C.; Chang, S.; Xie, Z.; Yu, D.-G.; Liu, Y.; Shao, J. Core–Shell Eudragit S100 Nanofibers Prepared via Triaxial Electrospinning to Provide a Colon-Targeted Extended Drug Release. Polymers 2020, 12, 2034. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Wang, M.; Zhang, F.; Liu, Y.; Liu, X.; Yu, D.-G.; Shen, H. Sheath-Separate-Core Nanocomposites Fabricated Using a Trifluid Electrospinning. Mater. Des. 2020, 192, 108782. [Google Scholar] [CrossRef]
- Chen, J.; Pan, S.; Zhou, J.; Lin, Z.; Qu, Y.; Glab, A.; Han, Y.; Richardson, J.J.; Caruso, F. Assembly of Bioactive Nanoparticles via Metal–Phenolic Complexation. Adv. Mater. 2021, 33, 2108624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yue, Y.-X.; Xu, L.; Wang, Y.; Geng, W.-C.; Li, J.-J.; Kong, X.; Zhao, X.; Zheng, Y.; Zhao, Y.; et al. Macrocyclic-Amphiphile-Based Self-Assembled Nanoparticles for Ratiometric Delivery of Therapeutic Combinations to Tumors. Adv. Mater. 2021, 33, 2007719. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yu, Z.; Liu, S.; Chen, Y.; Lv, Y.; Liu, Y.; Lin, C.; Ye, X.; Shi, Y.; Liu, M.; et al. Efficient Extraction of Trace Organochlorine Pesticides from Environmental Samples by a Polyacrylonitrile Electrospun Nanofiber Membrane Modified with Covalent Organic Framework. J. Hazard. Mater. 2022, 424, 127455. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Huang, Y.; Ye, J.; Xia, G.; Wang, G.; Dong, L.; Yang, Z.; Yu, X. Electrospun Carbon Nanofibers with In-Situ Encapsulated Ni Nanoparticles as Catalyst for Enhanced Hydrogen Storage of MgH2. J. Alloys Compd. 2021, 851, 156874. [Google Scholar] [CrossRef]
- Lv, H.; Cui, S.; Yang, Q.; Song, X.; Wang, D.; Hu, J.; Zhou, Y.; Liu, Y. AgNPs-Incorporated Nanofiber Mats: Relationship between AgNPs Size/Content, Silver Release, Cytotoxicity, and Antibacterial Activity. Mater. Sci. Eng. C 2021, 118, 111331. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Z.; Liu, L.; Gao, M.; Han, Z. A Stable Metal-Organic Framework Nanofibrous Membrane as Photocatalyst for Simultaneous Removal of Methyl Orange and Formaldehyde from Aqueous Solution. Colloids Surf. A 2021, 617, 126359. [Google Scholar] [CrossRef]
- Lee, J.; Jung, S.; Park, H.; Kim, J. Bifunctional ZIF-8 Grown Webs for Advanced Filtration of Particulate and Gaseous Matters: Effect of Charging Process on the Electrostatic Capture of Nanoparticles and Sulfur Dioxide. ACS Appl. Mater. Interfaces 2021, 13, 50401–50410. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Si, Y.; Yu, J.; Ding, B. Facile Access to Highly Flexible and Mesoporous Structured Silica Fibrous Membranes for Tetracyclines Removal. Chem. Eng. J. 2021, 417, 129211. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Zhang, L.; Xia, S.; Zhao, Y.; Yan, J.; Yu, J.; Ding, B. Synthesizing Superior Flexible Oxide Perovskite Ceramic Nanofibers by Precisely Controlling Crystal Nucleation and Growth. Small 2021, 11, 2106500. [Google Scholar] [CrossRef]
- Lee, S.S.; Bai, H.; Chua, S.C.; Lee, K.W.; Sun, D.D. Electrospun Bi3+/TiO2 Nanofibers for Concurrent Photocatalytic H2 and Clean Water Production from Glycerol under Solar Irradiation: A Systematic Study. J. Cleaner Prod. 2021, 298, 126671. [Google Scholar] [CrossRef]
- Xie, W.; Shi, Y.; Wang, Y.; Zheng, Y.; Liu, H.; Hu, Q.; Wei, S.; Gu, H.; Guo, Z. Electrospun Iron/Cobalt Alloy Nanoparticles on Carbon Nanofibers towards Exhaustive Electrocatalytic Degradation of Tetracycline in Wastewater. Chem. Eng. J. 2021, 405, 126585. [Google Scholar] [CrossRef]
- Chang, Y.; Dong, C.; Zhou, D.; Li, A.; Dong, W.; Cao, X.-Z.; Wang, G. Fabrication and Elastic Properties of TiO2 Nanohelix Arrays through a Pressure-Induced Hydrothermal Method. ACS Nano 2021, 15, 14174–14184. [Google Scholar] [CrossRef]
- Jung, K.-W.; Lee, S.Y.; Choi, J.-W.; Hwang, M.-J.; Shim, W.G. Synthesis of Mg–Al Layered Double Hydroxides-Functionalized Hydrochar Composite via an in Situ One-Pot Hydrothermal Method for Arsenate and Phosphate Removal: Structural Characterization and Adsorption Performance. Chem. Eng. J. 2021, 420, 129775. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Peng, C.; Yang, G.; Cao, Y.; Wang, H.; Peng, F.; Yu, H. One-Pot Synthesis of Ru/Nb2O5@Nb2C Ternary Photocatalysts for Water Splitting by Harnessing Hydrothermal Redox Reactions. Appl. Catal. B 2022, 303, 120910. [Google Scholar] [CrossRef]
- Küçük, Ö.; Teber, S.; Cihan Kaya, İ.; Akyıldız, H.; Kalem, V. Photocatalytic Activity and Dielectric Properties of Hydrothermally Derived Tetragonal BaTiO3 Nanoparticles Using TiO2 Nanofibers. J. Alloys Compd. 2018, 765, 82–91. [Google Scholar] [CrossRef]
- Mukhiya, T.; Tiwari, A.P.; Chhetri, K.; Kim, T.; Dahal, B.; Muthurasu, A.; Kim, H.Y. A Metal–Organic Framework Derived Cobalt Oxide/Nitrogen-Doped Carbon Nanotube Nanotentacles on Electrospun Carbon Nanofiber for Electrochemical Energy Storage. Chem. Eng. J. 2021, 420, 129679. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, H.J. Confinement of Zn-Mg-Al-Layered Double Hydroxide and α-Fe2O3 Nanorods on Hollow Porous Carbon Nanofibers: A Free-Standing Electrode for Solid-State Symmetric Supercapacitors. Chem. Eng. J. 2022, 429, 132345. [Google Scholar] [CrossRef]
- Yu, D.-G. Preface. Curr. Drug Deliv. 2021, 18, 2–3. [Google Scholar] [CrossRef]
- Wang, M.; Li, D.; Li, J.; Li, S.; Chen, Z.; Yu, D.-G.; Liu, Z.; Guo, J.Z. Electrospun Janus Zein–PVP Nanofibers Provide a Two-Stage Controlled Release of Poorly Water-Soluble Drugs. Mater. Des. 2020, 196, 109075. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Taskin, M.B.; Ahmad, T.; Wistlich, L.; Meinel, L.; Schmitz, M.; Rossi, A.; Groll, J. Bioactive Electrospun Fibers: Fabrication Strategies and a Critical Review of Surface-Sensitive Characterization and Quantification. Chem. Rev. 2021, 121, 11194–11237. [Google Scholar] [CrossRef] [PubMed]
- Jurak, M.; Wiącek, A.E.; Ładniak, A.; Przykaza, K.; Szafran, K. What Affects the Biocompatibility of Polymers? Adv. Colloid Interface Sci. 2021, 294, 102451. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, M.; Zhou, Y.; Sun, M.; Xie, Y.; Yu, D.-G. Comparisons of Antibacterial Performances between Electrospun Polymer@drug Nanohybrids with Drug-Polymer Nanocomposites. Adv. Compos. Hybrid Mater. 2022, 5. [Google Scholar] [CrossRef]
- Tuğcu-Demiröz, F.; Saar, S.; Kara, A.A.; Yıldız, A.; Tunçel, E.; Acartürk, F. Development and Characterization of Chitosan Nanoparticles Loaded Nanofiber Hybrid System for Vaginal Controlled Release of Benzydamine. Eur. J. Pharm. Sci. 2021, 161, 105801. [Google Scholar] [CrossRef]
- He, H.; Wu, M.; Zhu, J.; Yang, Y.; Ge, R.; Yu, D.-G. Engineered Spindles of Little Molecules Around Electrospun Nanofibers for Biphasic Drug Release. Adv. Fiber Mater. 2021, 3, 1–13. [Google Scholar] [CrossRef]
- Li, B.; Xia, X.; Chen, J.; Xia, D.; Xu, R.; Zou, X.; Wang, H.; Liang, C. Paclitaxel-Loaded Lignin Particle Encapsulated into Electrospun PVA/PVP Composite Nanofiber for Effective Cervical Cancer Cell Inhibition. Nanotechnology 2020, 32, 015101. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Mirzaei, S.; Gholami, M.H.; Hashemi, F.; Zabolian, A.; Raei, M.; Hushmandi, K.; Zarrabi, A.; Voelcker, N.H.; Aref, A.R.; et al. Hyaluronic Acid-Based Nanoplatforms for Doxorubicin: A Review of Stimuli-Responsive Carriers, Co-Delivery and Resistance Suppression. Carbohydr. Polym. 2021, 272, 118491. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, Y.; Yang, Z.; Zuo, G.; Zhang, Q.; Yao, F.; Wan, Y. Encapsulating Doxorubicin-Intercalated Lamellar Nanohydroxyapatite into PLGA Nanofibers for Sustained Drug Release. Curr. Appl. Phys. 2019, 19, 1204–1210. [Google Scholar] [CrossRef]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, W.; Sadeghi-Soureh, S.; Amirsaadat, S.; Pourpirali, R.; Alijani, S. Dual Drug Release Mechanisms through Mesoporous Silica Nanoparticle/Electrospun Nanofiber for Enhanced Anticancer Efficiency of Curcumin. J. Biomed. Mater. Res. Part A 2021, 1–15. [Google Scholar] [CrossRef]
- Samadzadeh, S.; Babazadeh, M.; Zarghami, N.; Pilehvar-Soltanahmadi, Y.; Mousazadeh, H. An Implantable Smart Hyperthermia Nanofiber with Switchable, Controlled and Sustained Drug Release: Possible Application in Prevention of Cancer Local Recurrence. Mater. Sci. Eng. C 2021, 118, 111384. [Google Scholar] [CrossRef]
- Croitoru, A.-M.; Karaçelebi, Y.; Saatcioglu, E.; Altan, E.; Ulag, S.; Aydoğan, H.K.; Sahin, A.; Motelica, L.; Oprea, O.; Tihauan, B.-M.; et al. Electrically Triggered Drug Delivery from Novel Electrospun Poly(Lactic Acid)/Graphene Oxide/Quercetin Fibrous Scaffolds for Wound Dressing Applications. Pharmaceutics 2021, 13, 957. [Google Scholar] [CrossRef]
- Li, D.; Wang, M.; Song, W.-L.; Yu, D.-G.; Bligh, S.W.A. Electrospun Janus Beads-On-A-String Structures for Different Types of Controlled Release Profiles of Double Drugs. Biomolecules 2021, 11, 635. [Google Scholar] [CrossRef]
- Gupta, N.; Kamath, S.M.; Rao, S.K.; Jaison, D.; Patil, S.; Gupta, N.; Arunachalam, K.D. Kaempferol Loaded Albumin Nanoparticles and Dexamethasone Encapsulation into Electrospun Polycaprolactone Fibrous Mat–Concurrent Release for Cartilage Regeneration. J. Drug Deliv. Sci. Technol. 2021, 64, 102666. [Google Scholar] [CrossRef]
- Chen, K.; Pan, H.; Ji, D.; Li, Y.; Duan, H.; Pan, W. Curcumin-Loaded Sandwich-like Nanofibrous Membrane Prepared by Electrospinning Technology as Wound Dressing for Accelerate Wound Healing. Mater. Sci. Eng. C 2021, 127, 112245. [Google Scholar] [CrossRef] [PubMed]
- Bardoňová, L.; Kotzianová, A.; Skuhrovcová, K.; Židek, O.; Bártová, T.; Kulhánek, J.; Hanová, T.; Mamulová Kutláková, K.; Vágnerová, H.; Krpatová, V.; et al. Antimicrobial Nanofibrous Mats with Controllable Drug Release Produced from Hydrophobized Hyaluronan. Carbohydr. Polym. 2021, 267, 118225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, X.; Cui, Y.; Cheng, K.; Tian, X.; Dong, M.; Liu, L. Silk Fibroin H-Fibroin/Poly(ε-Caprolactone) Core-Shell Nanofibers with Enhanced Mechanical Property and Long-Term Drug Release. J. Colloid Interface Sci. 2021, 593, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, E.; Kahrizi, D.; Arkan, E.; Hosseinabadi, S.; Nematpour, N. Preparation of Bio-Nano Bandage from Quince Seed Mucilage/ZnO Nanoparticles and Its Application for the Treatment of Burn. J. Mol. Liq. 2021, 339, 116598. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.; Zhang, W.; Wang, M.; Li, J.; Zhang, Y.; Li, H. Surface Modification of a Polylactic Acid Nanofiber Membrane by Zeolitic Imidazolate Framework-8 from Secondary Growth for Drug Delivery. J. Mater. Sci. 2020, 55, 15275–15287. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Yu, D.-G.; Liu, H.; Liu, Y.; Liu, P. Electrospun PVP-Core/PHBV-Shell Fibers to Eliminate Tailing Off for an Improved Sustained Release of Curcumin. Mol. Pharm. 2021, 18, 4170–4178. [Google Scholar] [CrossRef]
- Banerjee, A.; Jariwala, T.; Baek, Y.-K.; To, D.T.H.; Tai, Y.; Liu, J.; Park, H.; Myung, N.V.; Nam, J. Magneto- and Opto-Stimuli Responsive Nanofibers as a Controlled Drug Delivery System. Nanotechnology 2021, 32, 505101. [Google Scholar] [CrossRef]
- Do Pazo-Oubiña, F.; Alorda-Ladaria, B.; Gomez-Lobon, A.; Boyeras-Vallespir, B.; Santandreu-Estelrich, M.M.; Martorell-Puigserver, C.; Gomez-Zamora, M.; Ventayol-Bosch, P.; Delgado-Sanchez, O. Thermolabile Drug Storage in an Ambulatory Setting. Sci. Rep. 2021, 11, 5959. [Google Scholar] [CrossRef]
- Sedláček, O.; Černoch, P.; Kučka, J.; Konefal, R.; Štěpánek, P.; Vetrík, M.; Lodge, T.P.; Hrubý, M. Thermoresponsive Polymers for Nuclear Medicine: Which Polymer Is the Best? Langmuir 2016, 32, 6115–6122. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Yu, D.-G.; Yang, Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus Nanofibers Loaded with a Drug and Inorganic Nanoparticles as an Effective Antibacterial Wound Dressing. Mater. Sci. Eng. C 2020, 111, 110805. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Khoshnava, S.M.; Pagan, E.; Chen, X. Co-Incorporation of Graphene Oxide/Silver Nanoparticle into Poly-L-Lactic Acid Fibrous: A Route toward the Development of Cytocompatible and Antibacterial Coating Layer on Magnesium Implants. Mater. Sci. Eng. C 2020, 111, 110812. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Reda, M.M.; Klingner, A. Preparation and Characterization of Green Carboxymethylchitosan (CMCS)–Polyvinyl Alcohol (PVA) Electrospun Nanofibers Containing Gold Nanoparticles (AuNPs) and Its Potential Use as Biomaterials. Int. J. Biol. Macromol. 2020, 151, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.A.; Nguyen, V.-C.; Phan, T.N.H.; Le, V.T.; Vasseghian, Y.; Trubitsyn, M.A.; Nguyen, A.-T.; Chau, T.P.; Doan, V.-D. Novel Biogenic Silver and Gold Nanoparticles for Multifunctional Applications: Green Synthesis, Catalytic and Antibacterial Activity, and Colorimetric Detection of Fe(III) Ions. Chemosphere 2022, 287, 132271. [Google Scholar] [CrossRef]
- Li, Z.; Tian, C.; Jiao, D.; Li, J.; Li, Y.; Zhou, X.; Zhao, H.; Zhao, Y.; Han, X. Synergistic Effects of Silver Nanoparticles and Cisplatin in Combating Inflammation and Hyperplasia of Airway Stents. Bioact. Mater. 2022, 9, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; He, J.; Zhu, L.; Machmudah, S.; Wahyudiono; Kanda, H.; Goto, M. Synthesis of Hollow PVP/Ag Nanoparticle Composite Fibers via Electrospinning under a Dense CO2 Environment. Polymers 2022, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Jin, L.; Yu, F.; Wang, F.; Weng, Z.; Liu, J.; Han, Z.; Wang, X. ZnO/CPAN Modified Contact Lens with Antibacterial and Harmful Light Reduction Capabilities. Adv. Healthc. Mater. 2021, 10, 2100259. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, G.; Gao, Q.; Wang, X.; Wang, Y.; Xu, X.; Yan, W.; Shen, H. Sprayed Copper Peroxide Nanodots for Accelerating Wound Healing in a Multidrug-Resistant Bacteria Infected Diabetic Ulcer. Nanoscale 2021, 13, 15937–15951. [Google Scholar] [CrossRef]
- Shi, J.; Li, J.; Wang, Y.; Zhang, C.Y. TiO2-Based Nanosystem for Cancer Therapy and Antimicrobial Treatment: A Review. Chem. Eng. J. 2021, 431, 133714. [Google Scholar] [CrossRef]
- Villarreal-Gómez, L.J.; Pérez-González, G.L.; Bogdanchikova, N.; Pestryakov, A.; Nimaev, V.; Soloveva, A.; Cornejo-Bravo, J.M.; Toledaño-Magaña, Y. Antimicrobial Effect of Electrospun Nanofibers Loaded with Silver Nanoparticles: Influence of Ag Incorporation Method. J. Nanomater. 2021, 2021, e9920755. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Wu, F.; Sajjad, W.; Zhang, Q.; Khan, I.; Ullah, K.; Wang, W. Long-Term Exposure to High-Concentration Silver Nanoparticles Induced Toxicity, Fatality, Bioaccumulation, and Histological Alteration in Fish (Cyprinus Carpio). Environ. Sci. Eur. 2021, 33, 14. [Google Scholar] [CrossRef]
- Lemraski, E.G.; Jahangirian, H.; Dashti, M.; Khajehali, E.; Sharafinia, S.; Rafiee-Moghaddam, R.; Webster, T.J. Antimicrobial Double-Layer Wound Dressing Based on Chitosan/Polyvinyl Alcohol/Copper: In Vitro and in Vivo Assessment. Int. J. Nanomed. 2021, 16, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Feng, Y.; He, M.; Zhao, W.; Qiu, L.; Zhao, C. A Hierarchical Janus Nanofibrous Membrane Combining Direct Osteogenesis and Osteoimmunomodulatory Functions for Advanced Bone Regeneration. Adv. Funct. Mater. 2021, 31, 2008906. [Google Scholar] [CrossRef]
- Saatchi, A.; Arani, A.R.; Moghanian, A.; Mozafari, M. Cerium-Doped Bioactive Glass-Loaded Chitosan/Polyethylene Oxide Nanofiber with Elevated Antibacterial Properties as a Potential Wound Dressing. Ceram. Int. 2021, 47, 9447–9461. [Google Scholar] [CrossRef]
- Norouzi, M.A.; Montazer, M.; Harifi, T.; Karimi, P. Flower Buds like PVA/ZnO Composite Nanofibers Assembly: Antibacterial, in Vivo Wound Healing, Cytotoxicity and Histological Studies. Polym. Test. 2021, 93, 106914. [Google Scholar] [CrossRef]
- Khan, A.U.R.; Huang, K.; Jinzhong, Z.; Zhu, T.; Morsi, Y.; Aldalbahi, A.; El-Newehy, M.; Yan, X.; Mo, X. Exploration of the Antibacterial and Wound Healing Potential of a PLGA/Silk Fibroin Based Electrospun Membrane Loaded with Zinc Oxide Nanoparticles. J. Mater. Chem. B 2021, 9, 1452–1465. [Google Scholar] [CrossRef]
- Huang, C.; Dong, J.; Zhang, Y.; Chai, S.; Wang, X.; Kang, S.; Yu, D.; Wang, P.; Jiang, Q. Gold Nanoparticles-Loaded Polyvinylpyrrolidone/Ethylcellulose Coaxial Electrospun Nanofibers with Enhanced Osteogenic Capability for Bone Tissue Regeneration. Mater. Des. 2021, 212, 110240. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, H.; Zhang, H.; Xin, Q.; Zhang, H.; Liu, H.; Wu, M.; Zuo, L.; Luo, J.; Guo, Q.; et al. Heterogenous Hydrogel Mimicking the Osteochondral ECM Applied to Tissue Regeneration. J. Mater. Chem. B 2021, 9, 8646–8658. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, W.; Zhang, J.; Bártolo, P.; Gong, H.; Li, J. Electrospun Highly Porous Poly(L-Lactic Acid)-Dopamine-SiO2 Fibrous Membrane for Bone Regeneration. Mater. Sci. Eng. C 2020, 117, 111359. [Google Scholar] [CrossRef]
- Stahl, A.; Yang, Y.P. Regenerative Approaches for the Treatment of Large Bone Defects. Tissue Eng. Part B 2021, 27, 539–547. [Google Scholar] [CrossRef]
- Hassani, A.; Khoshfetrat, A.B.; Rahbarghazi, R.; Sakai, S. Collagen and Nano-Hydroxyapatite Interactions in Alginate-Based Microcapsule Provide an Appropriate Osteogenic Microenvironment for Modular Bone Tissue Formation. Carbohydr. Polym. 2022, 277, 118807. [Google Scholar] [CrossRef] [PubMed]
- Kermani, F.; Mollazadeh, S.; Kargozar, S.; Vahdati Khakhi, J. Improved Osteogenesis and Angiogenesis of Theranostic Ions Doped Calcium Phosphates (CaPs) by a Simple Surface Treatment Process: A State-of-the-Art Study. Mater. Sci. Eng. C 2021, 124, 112082. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Liao, C.; Huang, L.; Liang, R.; Zhao, J.; Zheng, L.; Su, W. Electrospun PCL/MoS2 Nanofiber Membranes Combined with NIR-Triggered Photothermal Therapy to Accelerate Bone Regeneration. Small 2021, 17, 2104747. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Shen, D.; Zhou, W.; Zheng, Y.; Kong, T.; Liu, X.; Wu, S.; Chu, P.K.; Zhao, Y.; Wu, J.; et al. Regulation of Extracellular Bioactive Cations in Bone Tissue Microenvironment Induces Favorable Osteoimmune Conditions to Accelerate in Situ Bone Regeneration. Bioact. Mater. 2021, 6, 2315–2330. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Mao, H.; Zhang, Y.; Zheng, L.; Yu, P.; Guo, Z.; Li, L.; Jiang, Q. PEGylated Gold Nanoparticles Promote Osteogenic Differentiation in in Vitro and in Vivo Systems. Mater. Des. 2021, 197, 109231. [Google Scholar] [CrossRef]
- Song, H.; Zhang, Y.; Zhang, Z.; Xiong, S.; Ma, X.; Li, Y. Hydroxyapatite/NELL-1 Nanoparticles Electrospun Fibers for Osteoinduction in Bone Tissue Engineering Application. Int. J. Nanomed. 2021, 16, 4321–4332. [Google Scholar] [CrossRef]
- Liu, W.; Bi, W.; Sun, Y.; Wang, L.; Yu, X.; Cheng, R.; Yu, Y.; Cui, W. Biomimetic Organic-Inorganic Hybrid Hydrogel Electrospinning Periosteum for Accelerating Bone Regeneration. Mater. Sci. Eng. C 2020, 110, 110670. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-H.; Li, X.-Y.; Zhang, W.-D.; Li, H.-H.; Wang, S.-G.; Wu, X.-L.; Su, Z.-M.; Zhang, J.-P.; Sun, H.-Z. Micron-Scaled MoS2/N-C Particles with Embedded Nano-MoS2: A High-Rate Anode Material for Enhanced Lithium Storage. Appl. Surf. Sci. 2019, 486, 519–526. [Google Scholar] [CrossRef]
- Wu, S.; Wang, J.; Jin, L.; Li, Y.; Wang, Z. Effects of Polyacrylonitrile/MoS2 Composite Nanofibers on the Growth Behavior of Bone Marrow Mesenchymal Stem Cells. ACS Appl. Nano Mater. 2018, 1, 337–343. [Google Scholar] [CrossRef]
- Kasper, M.; Ellenbogen, B.; Hardy, R.; Cydis, M.; Mojica-Santiago, J.; Afridi, A.; Spearman, B.S.; Singh, I.; Kuliasha, C.A.; Atkinson, E.; et al. Development of a Magnetically Aligned Regenerative Tissue-Engineered Electronic Nerve Interface for Peripheral Nerve Applications. Biomaterials 2021, 279, 121212. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ge, X.; Qian, Y.; Tang, H.; Song, J.; Qu, X.; Yue, B.; Yuan, W.-E. Electrospinning Multilayered Scaffolds Loaded with Melatonin and Fe3O4 Magnetic Nanoparticles for Peripheral Nerve Regeneration. Adv. Funct. Mater. 2020, 30, 2004537. [Google Scholar] [CrossRef]
- Morsink, M.; Severino, P.; Luna-Ceron, E.; Hussain, M.A.; Sobahi, N.; Shin, S.R. Effects of Electrically Conductive Nano-Biomaterials on Regulating Cardiomyocyte Behavior for Cardiac Repair and Regeneration. Acta Biomater. 2021, 126, S17427061(21)007716. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, X.; Li, B.; Huang, G.; Xu, F.; Zhang, X. Solvent-Free Fabrication of Carbon Nanotube/Silk Fibroin Electrospun Matrices for Enhancing Cardiomyocyte Functionalities. ACS Biomater. Sci. Eng. 2020, 6, 1630–1640. [Google Scholar] [CrossRef]
- He, C.; Liu, X.; Zhou, Z.; Liu, N.; Ning, X.; Miao, Y.; Long, Y.; Wu, T.; Leng, X. Harnessing Biocompatible Nanofibers and Silver Nanoparticles for Wound Healing: Sandwich Wound Dressing versus Commercial Silver Sulfadiazine Dressing. Mater. Sci. Eng. C 2021, 128, 112342. [Google Scholar] [CrossRef]
- Talikowska, M.; Fu, X.; Lisak, G. Application of Conducting Polymers to Wound Care and Skin Tissue Engineering: A Review. Biosens. Bioelectron. 2019, 135, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Li, R.; Liu, G.; Jia, W.; Sun, M.; Liu, Y.; Luo, Y.; Cheng, Z. Phase Separation-Based Electrospun Janus Nanofibers Loaded with Rana Chensinensis Skin Peptides/Silver Nanoparticles for Wound Healing. Mater. Des. 2021, 207, 109864. [Google Scholar] [CrossRef]
- Gu, Y.; Son, S.U.; Li, T.; Tan, B. Low-Cost Hypercrosslinked Polymers by Direct Knitting Strategy for Catalytic Applications. Adv. Funct. Mater. 2021, 31, 2008265. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Hypercrosslinked Porous Polymer Materials: Design, Synthesis, and Applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, Y.; Varyambath, A.; Kim, I. Guided Assembly of Well-Defined Hierarchical Nanoporous Polymers by Lewis Acid–Base Interactions. ACS Nano 2019, 13, 11753–11769. [Google Scholar] [CrossRef]
- Song, W.; Zhang, Y.; Yu, D.-G.; Tran, C.H.; Wang, M.; Varyambath, A.; Kim, J.; Kim, I. Efficient Synthesis of Folate-Conjugated Hollow Polymeric Capsules for Accurate Drug Delivery to Cancer Cells. Biomacromolecules 2021, 22, 732–742. [Google Scholar] [CrossRef]
- Petre, D.G.; Leeuwenburgh, S.C.G. The Use of Fibers in Bone Tissue Engineering. Tissue Eng. Part B Rev. 2021, 27. [Google Scholar] [CrossRef]
- Wei, C.; Feng, Y.; Che, D.; Zhang, J.; Zhou, X.; Shi, Y.; Wang, L. Biomaterials in Skin Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2021, 1–19. [Google Scholar] [CrossRef]
- Yahya, E.B.; Amirul, A.A.; Abdul Khalil, H.P.S.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; Atty Sofea, A.K.; Adnan, A.S. Insights into the Role of Biopolymer Aerogel Scaffolds in Tissue Engineering and Regenerative Medicine. Polymers 2021, 13, 1612. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-J.; Wei, S.-Y.; Lin, T.-Y.; Fang, L.; Hsieh, Y.-T.; Chen, Y.-C. A Strategy to Engineer Vascularized Tissue Constructs by Optimizing and Maintaining the Geometry. Acta Biomater. 2022, 138, 254–272. [Google Scholar] [CrossRef]
- Yu, D.G.; Wang, M.; Ge, R. Strategies for Sustained Drug Release from Electrospun Multi-layer Nanostructures. WIRES Nanomed. Nanobi. 2021, 14, e1772. [Google Scholar] [CrossRef] [PubMed]
- Kundrat, V.; Vykoukal, V.; Moravec, Z.; Simonikova, L.; Novotny, K.; Pinkas, J. Preparation of Polycrystalline Tungsten Nanofibers by Needleless Electrospinning. J. Alloys Compd. 2022, 900, 163542. [Google Scholar] [CrossRef]











| Influence Factors | Influence Results | Reason for Influence | Ref. | |
|---|---|---|---|---|
| System parameters | Polymer concentration | The higher the concentration, the coarser the fiber | As polymer concentration or molecular weight increases, so does solution viscosity. Greater entanglement between molecule chains and increased intermolecular Coulomb forces result from this condition. As a result, the fiber diameter expands. | [35] |
| The molecular weight of polymers | The higher the molecular weight, the thicker the fiber | [36,37] | ||
| Surface tension | The higher the surface tension, the finer the fiber | The droplet’s surface tension rises, and the jet must expend more energy to offset this negative effect. The speed of the jet slows down, requiring more time to stretch the fibers. As a result, the fiber diameter decreases. | [38] | |
| Conductivity | Conductivity increases within a reasonable range; fiber diameter decreases and increases again; fiber diameter is not controllable | The charge accumulates on the surface of the jet when the conductivity is increased. The fibers stretch more quickly in this state. As a result, the diameter of the fiber is lowered. The coulombic repulsion at the jet interface is intensified when the solution conductivity is raised further. Uncontrollable fiber diameter distribution results from the unstable bending whip effect. | [39,40,41] | |
| Process parameters | Voltage | The fiber diameter decreases with higher voltage and increases with higher voltage | As the voltage rises, the charge density on the jet’s surface rises in accumulation. The circumstance may lead to a significant effect of jet stretching. Therefore, the fiber diameter decreases. The flow rate at the spinneret, on the other hand, increases as the voltage is raised more. Instead, the diameter of the fiber rises. | [42] |
| Flow rate | The flow rate increases; the fiber diameter increases; and further increases may result in droplets | The solution at the spinneret rises as the flow rate increases. This condition causes the fiber’s diameter to thicken. When the flow rate is too fast, the solution’s gravity causes it to trickle straight down. | [43,44] | |
| Receiving distance | Acceptance distance enlarges and fiber diameter reduces | The additional receiving distance gives the jet more time to extend. The fiber’s diameter shrinks in this circumstance. | [45] | |
| Environmental factors | Temperature | Within a reasonable range, the fiber diameter decreases as the temperature increases | The temperature has the greatest influence on the viscosity of the solution. The viscosity of the solution reduces as the temperature rises. The intermolecular Coulomb force is lessened in this scenario. | [46] |
| Humidity | Humidity increases and grooves appear on the fiber surface | When humidity is too high, fiber production is accelerated. Water droplet condensation on the fiber surface. Wrinkles occur on the surface of the fibers as a result of this process. | [47] |
| Polymers | NPs | NNHs | Preparation Methods | Bacterial Strains | Evaluation Methodology | Antibacterial Ability | Ref. |
|---|---|---|---|---|---|---|---|
| PVA/CS | CuNPs | PVA/CS/Cu | Co-blending | S. Aureus (ATCC 25923); B. cereus (ATC 11788); E. coli (ATCC 35218); P. aeruginosa (ATCC 49189) | Antibacterial Circle | The size of the inhibition circle is: S. Aureus (15.6 ± 1.1 mm); B. cereus (29.6 ± 0.42 mm); E. coli (13.3 ± 0.8 mm); P. aeruginosa (10 ± 1 mm) | [144] |
| GEL/PCL/P(DMC-AMA) | nHAP | JGM | Co-blending | E. Coli and S. Aureus | CFU Counting | The bacterial viability of S. aureus after 6 h was 0.1%. | [145] |
| Starch | AgNPs | starch/AgNPs | In-situ synthesis | E. Coli (ATCC 35218); S. Aureus (ATCC 29213) | Disc Diffusion-8mm | E. coli (9.7 mm); S. Aureus (10.2 mm) | [96] |
| PMMA | ZnO nanorods/AgNPs | PMMA/ZnO-Ag NF | Co-blending, in situ synthesis | E. coli (ATCC 25922); S. aureus (ATCC 25923) | Disc Diffusion-6mm | E. coli (7–17 mm); S. Aureus (8.5–18.5 mm) | [81] |
| CH/PEO | 8Ce-BG | CH-PEO-(8Ce-BG) | Co-blending | E. Coli and S. Aureus | Flat Counting Method | E. coli activity was only 55.3% | [146] |
| PLLA | GO-Ag | PLLA-GO-AgNPs | Co-blending | S. aureus (ATCC 12600); E. coli (ATCC 9637) | Antibacterial Circle | 3.01 mm–4.62 mm | [133] |
| PVP K90/EC | CIP/AgNPs | PVP-CIP//EC-AgNPs | Co-blending | S. aureus (ATCC 27853); E. coli (ATCC 25922) | Antibacterial Circle | 24 h, E. coli (17.8 ± 0.6mm mm); S. Aureus (21.9 ± 0.6 mm) | [132] |
| PVA | ZnO | PVA/ZnO | Self-assembly | S. Aureus (ATCC25923); E. coli (ATCC25922) | MIC method | E. coli (62.5 μg/mL); S. Aureus (250 μg/mL) | [147] |
| PLGA/SF | ZnO | PSZ | Co-blending | E. Coli and S. Aureus | turbidity measurement method | PSZ antibacterial activity against S. Aureus: 45.1–100% | [148] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Song, W.; Tang, Y.; Xu, X.; Huang, Y.; Yu, D. Polymer-Based Nanofiber–Nanoparticle Hybrids and Their Medical Applications. Polymers 2022, 14, 351. https://doi.org/10.3390/polym14020351
Zhang M, Song W, Tang Y, Xu X, Huang Y, Yu D. Polymer-Based Nanofiber–Nanoparticle Hybrids and Their Medical Applications. Polymers. 2022; 14(2):351. https://doi.org/10.3390/polym14020351
Chicago/Turabian StyleZhang, Mingxin, Wenliang Song, Yunxin Tang, Xizi Xu, Yingning Huang, and Dengguang Yu. 2022. "Polymer-Based Nanofiber–Nanoparticle Hybrids and Their Medical Applications" Polymers 14, no. 2: 351. https://doi.org/10.3390/polym14020351
APA StyleZhang, M., Song, W., Tang, Y., Xu, X., Huang, Y., & Yu, D. (2022). Polymer-Based Nanofiber–Nanoparticle Hybrids and Their Medical Applications. Polymers, 14(2), 351. https://doi.org/10.3390/polym14020351