Processing Compostable PLA/Organoclay Bionanocomposite Foams by Supercritical CO2 Foaming for Sustainable Food Packaging
Abstract
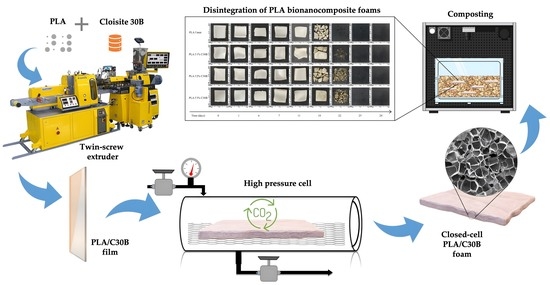
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLA Films and Bionanocomposites
2.3. Supercritical Foaming of PLA Composites
2.4. Film and Foam Characterization
2.4.1. Viscosity Molecular Weight
2.4.2. Morphological Analysis
2.4.3. Fourier Transform Infrared (FTIR)—Attenuated Total Reflectance (ATR) Spectroscopy
2.4.4. X-ray Diffraction (XRD)
2.4.5. Thermal Properties
2.4.6. Mechanical Properties
2.4.7. Water Absorption Analysis
2.4.8. Disintegration under Standard Composting Conditions
2.5. Statistical Analysis
3. Results
3.1. Processing of PLA Bionanocomposite Films and Foams
3.2. Viscosity Molecular Weight
3.3. Morphological Results
3.4. FTIR Spectra Results
3.5. X-ray Diffraction (XRD)
3.6. Thermal Properties
3.7. Mechanical Properties
3.8. Water Absorption Analysis
3.9. Disintegration under Composting Conditions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghoshal, G. Chapter 10—Recent Trends in Active, Smart, and Intelligent Packaging for Food Products. In Handbook of Food Bioengineering; Grumezescu, A.M., Holban, A.M.B.T.-F.P.P., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 343–374. ISBN 978-0-12-811516-9. [Google Scholar]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves de Moura, I.G.; de Sá, A.V.; Abreu, A.S.; Lemos, M.; Machado, A.V. 7—Bioplastics from agro-wastes for food packaging applications. In Food Packaging; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 223–263. ISBN 978-0-12-804302-8. [Google Scholar]
- Jabeen, N.; Majid, I.; Nayik, G.A. Bioplastics and food packaging: A review. Cogent Food Agric. 2015, 1, 1117749. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic Acid Technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- García-Arroyo, P.; Arrieta, M.P.; Garcia-Garcia, D.; Cuervo-Rodríguez, R.; Fombuena, V.; Mancheño, M.J.; Segura, J.L. Plasticized poly (lactic acid) reinforced with antioxidant covalent organic frameworks (COFs) as novel nanofillers designed for non-migrating active packaging applications. Polymer 2020, 196, 122466. [Google Scholar] [CrossRef]
- Chauvet, M.; Sauceau, M.; Baillon, F.; Fages, J. Foaming PLA with Thermoplastic Starch by Extrusion Assisted by Supercritical CO2, International Society for the Advancement of Supercritical Fluids (ISASF). In Proceedings of the 16th European Meeting on Supercritical Fluids (EMSF 2017), Lisbonne, Portugal, 25–28 April 2017. [Google Scholar]
- Nofar, M.; Park, C.B. Poly (lactic acid) foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Milovanovic, S.; Markovic, D.; Mrakovic, A.; Kuska, R.; Zizovic, I.; Frerich, S.; Ivanovic, J. Supercritical CO2—assisted production of PLA and PLGA foams for controlled thymol release. Mater. Sci. Eng. C 2019, 99, 394–404. [Google Scholar] [CrossRef]
- Zimmermann, M.V.G.; da Silva, M.P.; Zattera, A.J.; Santana, R.M.C. Poly(lactic acid) foams reinforced with cellulose micro and nanofibers and foamed by chemical blowing agents. J. Cell. Plast. 2018, 54, 577–596. [Google Scholar] [CrossRef]
- Standau, T.; Zhao, C.; Murillo Castellón, S.; Bonten, C.; Altstädt, V. Chemical Modification and Foam Processing of Polylactide (PLA). Polymers 2019, 11, 306. [Google Scholar] [CrossRef] [Green Version]
- Villamil Jiménez, J.A.; Le Moigne, N.; Bénézet, J.C.; Sauceau, M.; Sescousse, R.; Fages, J. Foaming of PLA composites by supercritical fluid-assisted processes: A review. Molecules 2020, 25, 3408. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; Galotto, M.J.; Guarda, A.; Julio, R. Supercritical impregnation for food applications: A review of the effect of the operational variables on the active compound loading. Crit. Rev. Food Sci. Nutr. 2019, 60, 1290–1301. [Google Scholar] [CrossRef]
- Wang, J.; Chai, J.; Wang, G.; Zhao, J.; Zhang, D.; Li, B.; Zhao, H.; Zhao, G. Strong and thermally insulating polylactic acid/glass fiber composite foam fabricated by supercritical carbon dioxide foaming. Int. J. Biol. Macromol. 2019, 138, 144–155. [Google Scholar] [CrossRef]
- Morlin, B.; Litauszki, K.; Petrény, R.; Kmetty, Á.; Mészáros, L. Characterization of polylactic acid-based nanocomposite foams with supercritical CO2. Meas. J. Int. Meas. Confed. 2021, 178, 109385. [Google Scholar] [CrossRef]
- Nofar, M. Effects of nano-/micro-sized additives and the corresponding induced crystallinity on the extrusion foaming behavior of PLA using supercritical CO2. Mater. Des. 2016, 101, 24–34. [Google Scholar] [CrossRef]
- Lee, S.T. Polymeric Foams: Innovations in Processes, Technologies, and Products; Taylor & Francis: Boca Raton, FL, USA, 2017; ISBN 9781498738897. [Google Scholar]
- Iannace, S.; Park, C.B. Biofoams- Science and Applications of bio-Based Cellular and Porous Materials, 1st ed.; Taylor & Francis: Boca Raton, FL, USA, 2016; ISBN 9781466561809. [Google Scholar]
- Arrieta, M.P.; Fortunati, E.; Burgos, N.; Peltzer, M.A.; López, J.; Peponi, L. Chapter 7—Nanocellulose-Based Polymeric Blends for Food Packaging Applications; Puglia, D., Fortunati, E., Kenny, J., Eds.; William Andrew Publishing: New York, NY, USA, 2016; pp. 205–252. ISBN 978-0-323-44248-0. [Google Scholar]
- Molinaro, S.; Cruz Romero, M.; Boaro, M.; Sensidoni, A.; Lagazio, C.; Morris, M.; Kerry, J. Effect of nanoclay-type and PLA optical purity on the characteristics of PLA-based nanocomposite films. J. Food Eng. 2013, 117, 113–123. [Google Scholar] [CrossRef]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/organoclay bionanocomposites impregnated with thymol and cinnamaldehyde by supercritical impregnation for active and sustainable food packaging. Compos. Part B Eng. 2019, 176, 107336. [Google Scholar] [CrossRef]
- Yeh, S.-K.; Liu, Y.-C.; Chu, C.-C.; Chang, K.-C.; Wang, S.-F. Mechanical Properties of Microcellular and Nanocellular Thermoplastic Polyurethane Nanocomposite Foams Created Using Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2017, 56, 8499–8507. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Sinha Ray, S.; Okamoto, M.; Ogami, A.; Yamada, K.; Ueda, K. Well-controlled biodegradable nanocomposite foams: From microcellular to nanocellular. Macromol. Rapid Commun. 2003, 24, 457–461. [Google Scholar] [CrossRef]
- Chen, L.; Rende, D.; Schadler, L.S.; Ozisik, R. Polymer nanocomposite foams. J. Mater. Chem. A 2013, 1, 3837–3850. [Google Scholar] [CrossRef]
- Qiu, Y.; Lv, Q.; Wu, D.; Xie, W.; Peng, S.; Lan, R.; Xie, H. Cyclic tensile properties of the polylactide nanocomposite foams containing cellulose nanocrystals. Cellulose 2018, 25, 1795–1807. [Google Scholar] [CrossRef]
- Borkotoky, S.S.; Dhar, P.; Katiyar, V. Biodegradable poly (lactic acid)/Cellulose nanocrystals (CNCs) composite microcellular foam: Effect of nanofillers on foam cellular morphology, thermal and wettability behavior. Int. J. Biol. Macromol. 2018, 106, 433–446. [Google Scholar] [CrossRef]
- Zafar, M.T.; Kumar, S.; Singla, R.K.; Maiti, S.N.; Ghosh, A.K. Surface Treated Jute Fiber Induced Foam Microstructure Development in Poly(lactic acid)/Jute Fiber Biocomposites and their Biodegradation Behavior. Fibers Polym. 2018, 19, 648–659. [Google Scholar] [CrossRef]
- Rizvi, R.; Cochrane, B.; Naguib, H.; Lee, P.C. Fabrication and characterization of melt-blended polylactide-chitin composites and their foams. J. Cell. Plast. 2011, 47, 283–300. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Zhou, Y.; Zhang, Y.; Li, Q.; Shen, C. Fabrication of open-porous PCL/PLA tissue engineering scaffolds and the relationship of foaming process, morphology, and mechanical behavior. Polym. Adv. Technol. 2019, 30, 2539–2548. [Google Scholar] [CrossRef]
- Chai, J.; Wang, G.; Zhao, J.; Zhang, A.; Shi, Z.; Wei, C.; Zhao, G. Microcellular PLA/PMMA foam fabricated by CO2 foaming with outstanding shape-memory performance. J. CO2 Util. 2021, 49, 101553. [Google Scholar] [CrossRef]
- Li, B.; Zhao, G.; Wang, G.; Zhang, L.; Gong, J.; Shi, Z. Biodegradable PLA/PBS open-cell foam fabricated by supercritical CO2 foaming for selective oil-adsorption. Sep. Purif. Technol. 2021, 257, 117949. [Google Scholar] [CrossRef]
- Jeong, E.J.; Park, C.K.; Kim, S.H. Fabrication of microcellular polylactide/modified silica nanocomposite foams. J. Appl. Polym. Sci. 2019, 137, 48616. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Martín-Alfonso, J.E. Thermal, thermo-oxidative and thermomechanical degradation of PLA: A comparative study based on rheological, chemical and thermal properties. Polym. Degrad. Stab. 2018, 150, 37–45. [Google Scholar] [CrossRef]
- Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting Conditions in a Laboratory-Scale Test; ISO: Geneva, Switzerland, 2016; No. 20200:2015.
- Gil-Castell, O.; Badia, J.D.; Ingles-Mascaros, S.; Teruel-Juanes, R.; Serra, A.; Ribes-Greus, A. Polylactide-based self-reinforced composites biodegradation: Individual and combined influence of temperature, water and compost. Polym. Degrad. Stab. 2018, 158, 40–51. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Perdiguero, M.; Fiori, S.; Kenny, J.M.; Peponi, L. Biodegradable electrospun PLA-PHB fibers plasticized with oligomeric lactic acid. Polym. Degrad. Stab. 2020, 179, 109226. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Iannoni, A.; Barbale, M.; Zaccheo, S.; Scavone, M.; Visai, L.; Kenny, J.M. New multifunctional poly(lactide acid) composites: Mechanical, antibacterial, and degradation properties. J. Appl. Polym. Sci. 2012, 124, 87–98. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Rayón, E.; Jiménez, A. Disintegrability under composting conditions of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2014, 108, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Iturrondobeitia, M.; Okariz, A.; Guraya, T.; Zaldua, A.M.; Ibarretxe, J. Influence of the processing parameters and composition on the thermal stability of PLA/nanoclay bio-nanocomposites. J. Appl. Polym. Sci. 2014, 131, 9120–9127. [Google Scholar] [CrossRef]
- Zhou, Q.; Xanthos, M. Nanosize and microsize clay effects on the kinetics of the thermal degradation of polylactides. Polym. Degrad. Stab. 2009, 94, 327–338. [Google Scholar] [CrossRef]
- Matuana, L.M.; Diaz, C.A. Study of cell nucleation in microcellular poly (lactic acid) foamed with supercritical CO2 through a continuous-extrusion process. Ind. Eng. Chem. Res. 2010, 49, 2186–2193. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; López de Dicastillo, C.; Velásquez, E.; Villegas, C.; Faba, S.; Rivera, P.; Guarda, A.; Romero, J.; Galotto, M.J. Foaming with scCO2 and Impregnation with Cinnamaldehyde of PLA Nanocomposites for Food Packaging. Processes 2022, 10, 376. [Google Scholar] [CrossRef]
- Tsivintzelis, I.; Sanxaridou, G.; Pavlidou, E.; Panayiotou, C. Foaming of Polymers with Supercritical Fluids: A Thermodynamic Investigation. J. Supercrit. Fluids 2016, 110, 240–250. [Google Scholar] [CrossRef]
- Okolieocha, C.; Raps, D.; Subramaniam, K.; Altstädt, V. Microcellular to nanocellular polymer foams: Progress (2004–2015) and future directions—A review. Eur. Polym. J. 2015, 73, 500–519. [Google Scholar] [CrossRef]
- Velásquez, E.; Rojas, A.; Piña, C.; Galotto, M.J.; de Dicastillo, C.L. Development of bilayer biodegradable composites containing cellulose nanocrystals with antioxidant properties. Polymers 2019, 11, 1945. [Google Scholar] [CrossRef] [Green Version]
- Moliner, C.; Finocchio, E.; Arato, E.; Ramis, G.; Lagazzo, A. Influence of the degradation medium on water uptake, morphology, and chemical structure of Poly (Lactic Acid)-Sisal bio-composites. Materials 2020, 13, 3974. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Zhou, C.; Li, H.; Zhang, W.; Li, J.; Huang, S.; Meng, Y.; De Claville Christiansen, J.; Yu, D.; Wu, Z.; Jiang, S. Thermal strain-induced cold crystallization of amorphous poly (lactic acid). Cryst. Eng. Commun. 2016, 18, 3237–3246. [Google Scholar] [CrossRef] [Green Version]
- Hedayati, F.; Moshiri-Gomchi, N.; Assaran-Ghomi, M.; Sabahi, S.; Bahri-Laleh, N.; Mehdipour-Ataei, S.; Mokhtari-Aliabad, J.; Mirmohammadi, S.A. Preparation and properties of enhanced nanocomposites based on PLA/PC blends reinforced with silica nanoparticles. Polym. Adv. Technol. 2020, 31, 566–573. [Google Scholar] [CrossRef]
- Najafi, N.; Heuzey, M.C.; Carreau, P.J. Polylactide (PLA)-clay nanocomposites prepared by melt compounding in the presence of a chain extender. Compos. Sci. Technol. 2012, 72, 608–615. [Google Scholar] [CrossRef]
- Pluta, M.; Paul, M.A.; Alexandre, M.; Dubois, P. Plasticized polylactide/clay nanocomposites. I. The role of filler content and its surface organo-modification on the physico-chemical properties. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 299–311. [Google Scholar] [CrossRef]
- Barkoula, N.M.; Alcock, B.; Cabrera, N.O.; Peijs, T. Flame-Retardancy Properties of Intumescent Ammonium Poly (Phosphate) and Mineral Filler Magnesium Hydroxide in Combination with Graphene. Polym. Polym. Compos. 2008, 16, 101–113. [Google Scholar] [CrossRef]
- Xiao, H.W.; Li, P.; Ren, X.; Jiang, T.; Yeh, J.-T. Isothermal crystallization kinetics and crystal structure of poly(lactic acid): Effect of triphenyl phosphate and talc. J. Appl. Polym. Sci. 2010, 118, 3558–3569. [Google Scholar] [CrossRef]
- Scarfato, P.; Di Maio, L.; Milana, M.R.; Giamberardini, S.; Denaro, M.; Incarnato, L. Performance properties, lactic acid specific migration and swelling by simulant of biodegradable poly (lactic acid)/nanoclay multilayer films for food packaging. Food Addit. Contam. -Part A Chem. Anal. Control. Expo. Risk Assess. 2017, 34, 1730–1742. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly (ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Tang, N.; Lei, D.; Huang, D.; Xiao, R. Mechanical performance of polystyrene foam (EPS): Experimental and numerical analysis. Polym. Test. 2019, 73, 359–365. [Google Scholar] [CrossRef]
- Agüero, Á.; Lascano, D.; Garcia-Sanoguera, D.; Fenollar, O.; Torres-Giner, S. Valorization of linen processing by-products for the development of injection-molded green composite pieces of polylactide with improved performance. Sustainability 2020, 12, 652. [Google Scholar] [CrossRef] [Green Version]
- Kale, G.; Auras, R.; Singh, S.P. Degradation of commercial biodegradable packages under real composting and ambient exposure conditions. J. Polym. Environ. 2006, 14, 317–334. [Google Scholar] [CrossRef]
- Sessini, V.; Arrieta, M.P.; Raquez, J.-M.; Dubois, P.; Kenny, J.M.; Peponi, L. Thermal and composting degradation of EVA/Thermoplastic starch blends and their nanocomposites. Polym. Degrad. Stab. 2019, 159, 184–198. [Google Scholar] [CrossRef]















| Sample | η (mL g−1) | (g mol−1) |
|---|---|---|
| films | ||
| PLA neat | 133.55 | 165,000 |
| PLA 1% C30B | 119.64 | 140,200 |
| PLA 2% C30B | 120.89 | 142,000 |
| PLA 3% C30B | 111.02 | 125,400 |
| foams | ||
| PLAf neat | 121.72 | 144,750 |
| PLAf 1% C30B | 113.89 | 129,950 |
| PLAf 2% C30B | 105.82 | 117,750 |
| PLAf 3% C30B | 110.62 | 123,050 |
| Sample | d (μm) | (nm) | NC (× 1011 cell/cm3) | Vf |
|---|---|---|---|---|
| PLAf neat | 26.47 ± 6.11 a | 376 ± 119 a | 4.9 | 0.90 |
| PLAf 1% C30B | 23.35 ± 6,71 b | 395 ± 129 a | 7.1 | 0.91 |
| PLAf 2% C30B | 26.69 ± 6.52 a | 421 ± 136 a | 4.7 | 0.89 |
| PLAf 3% C30B | 21.58 ± 3.84 b | 486 ± 172 b | 8.2 | 0.83 |
| Sample | Td, 5% (°C) | Tmax (°C) |
|---|---|---|
| films | ||
| PLA neat | 333.9 | 363.9 |
| PLA1% C30B | 332.1 | 366.4 |
| PLA2% C30B | 331.7 | 365.1 |
| PLA3% C30B | 330.0 | 365.3 |
| foams | ||
| PLAf neat | 306.4 | 356.7 |
| PLAf 1% C30B | 330.5 | 364.2 |
| PLAf 2% C30B | 332.9 | 366.1 |
| PLAf 3% C30B | 332.3 | 365.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faba, S.; Arrieta, M.P.; Agüero, Á.; Torres, A.; Romero, J.; Rojas, A.; Galotto, M.J. Processing Compostable PLA/Organoclay Bionanocomposite Foams by Supercritical CO2 Foaming for Sustainable Food Packaging. Polymers 2022, 14, 4394. https://doi.org/10.3390/polym14204394
Faba S, Arrieta MP, Agüero Á, Torres A, Romero J, Rojas A, Galotto MJ. Processing Compostable PLA/Organoclay Bionanocomposite Foams by Supercritical CO2 Foaming for Sustainable Food Packaging. Polymers. 2022; 14(20):4394. https://doi.org/10.3390/polym14204394
Chicago/Turabian StyleFaba, Simón, Marina P. Arrieta, Ángel Agüero, Alejandra Torres, Julio Romero, Adrián Rojas, and María José Galotto. 2022. "Processing Compostable PLA/Organoclay Bionanocomposite Foams by Supercritical CO2 Foaming for Sustainable Food Packaging" Polymers 14, no. 20: 4394. https://doi.org/10.3390/polym14204394
APA StyleFaba, S., Arrieta, M. P., Agüero, Á., Torres, A., Romero, J., Rojas, A., & Galotto, M. J. (2022). Processing Compostable PLA/Organoclay Bionanocomposite Foams by Supercritical CO2 Foaming for Sustainable Food Packaging. Polymers, 14(20), 4394. https://doi.org/10.3390/polym14204394