Sources, Chemical Functionalization, and Commercial Applications of Nanocellulose and Nanocellulose-Based Composites: A Review
Abstract
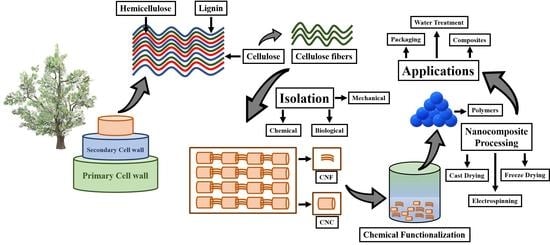
:1. Introduction
2. Nanocellulose and Its Various Sources
3. Nanocellulose Extraction Processes
3.1. Biomass Treatment for Nanocellulose Extraction
3.2. Nanocellulose Isolation
3.3. BNC Extraction
4. Chemical Treatment of Nanocellulose
5. Nanocellulose Composites and Their Processing
6. Applications of Nanocellulose and Its Composites
6.1. Nanocellulose Based Paper
6.2. Biomedical Applications
6.3. Food Packaging
6.4. Water Treatment
6.5. Coatings
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karim, M.R.A.; Tahir, D.; Haq, E.U.; Hussain, A.; Malik, M.S. Natural fibres as promising environmental-friendly reinforcements for polymer composites. Polym. Polym. Compos. 2020, 29, 277–300. [Google Scholar] [CrossRef]
- Tan, K.W.; Heo, S.K.; Foo, M.L.; Chew, I.M.L.; Yoo, C.K. An insight into nanocellulose as soft condensed matter: Challenge and future prospective toward environmental sustainability. Sci. Total Environ. 2019, 650, 1309–1326. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Aitomäki, Y.; Berglund, L.A.; Oksman, K.; Bismarck, A. On the use of nanocellulose as reinforcement in polymer matrix composites. Compos. Sci. Technol. 2014, 105, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, T.; Belete, A.; Syrowatka, F.; Neubert, R.H.H.; Gebre-Mariam, T. Extraction and characterization of celluloses from various plant byproducts. Int. J. Biol. Macromol. 2020, 158, 1248–1258. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Ul-Islam, M.; Kamal, T.; Ahmad, M.W.; Abbas, Y.; Manan, S.; Ullah, M.W.; Yang, G. Ex situ development and characterization of green antibacterial bacterial cellulose-based composites for potential biomedical applications. Adv. Compos. Hybrid Mater. 2022, 5, 307–321. [Google Scholar] [CrossRef]
- Stepanova, M.; Korzhikova-Vlakh, E. Modification of Cellulose Micro- and Nanomaterials to Improve Properties of Aliphatic Polyesters/Cellulose Composites: A Review. Polymers 2022, 14, 1477. [Google Scholar] [CrossRef]
- Zhou, L.; Ke, K.; Yang, M.B.; Yang, W. Recent progress on chemical modification of cellulose for high mechanical-performance Poly(lactic acid)/Cellulose composite: A review. Compos. Commun. 2021, 23, 100548. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Abdul Karim, M.R.; Tahir, D.; Hussain, A.; Ul Haq, E.; Khan, K.I. Sodium carbonate treatment of fibres to improve mechanical and water absorption characteristics of short bamboo natural fibres reinforced polyester composite. Plast. Rubber Compos. 2020, 49, 425–433. [Google Scholar] [CrossRef]
- Abdul Karim, M.R.; Tahir, D.; Khan, K.I.; Hussain, A.; Haq, E.U.; Malik, M.S. Improved mechanical and water absorption properties of epoxy-bamboo long natural fibres composites by eco-friendly Na2CO3 treatment. Plast. Rubber Compos. 2022, 22, 1–14. [Google Scholar] [CrossRef]
- Dufresne, A. Potential of nanocellulose as a reinforcing phase for polymers. J. Sci. Technol. For. Prod. Process. 2012, 2, 6–16. [Google Scholar]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Bhat, A.H.; Ireana Yusra, A.F. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Ferrer, A.; Pal, L.; Hubbe, M. Nanocellulose in packaging: Advances in barrier layer technologies. Ind. Crops Prod. 2017, 95, 574–582. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Dufresne, A. Chapter 1. Nanocellulose: Potential Reinforcement in Composites. In Natural Polymers: Volume 2: Nanocomposites; Maya, J., John, S.T., Eds.; RSC Publishing: Cambridge, UK, 2012; pp. 1–32. [Google Scholar]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa, A., Jr.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef] [Green Version]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Shi, Z.; Gauthier, M.; Yang, G. Bacterial cellulose: Molecular regulation of biosynthesis, supramolecular assembly, and tailored structural and functional properties. Prog. Mater. Sci. 2022, 129, 100972. [Google Scholar] [CrossRef]
- Haafiz, M.K.M.; Hassan, A.; Zakaria, Z.; Inuwa, I.M. Isolation and characterization of cellulose nanowhiskers from oil palm biomass microcrystalline cellulose. Carbohydr. Polym. 2014, 103, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mandal, T.; Goswami, S. Cellulose nanofibers from rice straw: Process development for improved delignification and better crystallinity index. Trends Carbohydr. Res. 2017, 9, 16–27. [Google Scholar]
- Dos Santos, R.M.; Flauzino Neto, W.P.; Silvério, H.A.; Martins, D.F.; Dantas, N.O.; Pasquini, D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind. Crops Prod. 2013, 50, 707–714. [Google Scholar] [CrossRef]
- Phanthong, P.; Ma, Y.; Guan, G.; Abudula, A. Extraction of Nanocellulose from Raw Apple Stem. J. Jpn. Inst. Energy. 2015, 94, 787–793. [Google Scholar] [CrossRef] [Green Version]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; Kargarzadeh, H., Ahmad, I., Thomas, A.D.S., Eds.; John Wiley & Sons, Ltd.: Weinheim, Germany, 2017; pp. 1–49. [Google Scholar]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Dong, X.M.; Revol, J.F.; Gray, D.G. Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 1998, 5, 19–32. [Google Scholar] [CrossRef]
- Maiti, S.; Jayaramudu, J.; Das, K.; Reddy, S.M.; Sadiku, R.; Ray, S.S.; Liu, D. Preparation and characterization of nano-cellulose with new shape from different precursor. Carbohydr. Polym. 2013, 98, 562–567. [Google Scholar] [CrossRef]
- Das, K.; Ray, D.; Bandyopadhyay, N.R.; Ghosh, T.; Mohanty, A.K.; Misra, M. A study of the mechanical, thermal and morphological properties of microcrystalline cellulose particles prepared from cotton slivers using different acid concentrations. Cellulose 2009, 16, 783–793. [Google Scholar] [CrossRef]
- Iranmahboob, J.; Nadim, F.; Monemi, S. Optimizing acid-hydrolysis: A critical step for production of ethanol from mixed wood chips. Biomass Bioenergy 2002, 22, 401–404. [Google Scholar] [CrossRef]
- Hamelinck, C.N.; Van Hooijdonk, G.; Faaij, A.P.C. Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 2005, 28, 384–410. [Google Scholar] [CrossRef]
- Wang, N.; Ding, E.; Cheng, R. Thermal degradation behaviors of spherical cellulose nanocrystals with sulfate groups. Polymer 2007, 48, 3486–3493. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Wahlström, R.M.; Suurnäkki, A. Enzymatic hydrolysis of lignocellulosic polysaccharides in the presence of ionic liquids. Green Chem. 2015, 17, 694–714. [Google Scholar] [CrossRef] [Green Version]
- Moniruzzaman, M.; Ono, T. Separation and characterization of cellulose fibers from cypress wood treated with ionic liquid prior to laccase treatment. Bioresour. Technol. 2013, 127, 132–137. [Google Scholar] [CrossRef]
- Li, J.; Wei, X.; Wang, Q.; Chen, J.; Chang, G.; Kong, L.; Su, J.; Liu, Y. Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr. Polym. 2012, 90, 1609–1613. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, K.; Hu, C.; Zhang, Z.; Chen, N.; Zhang, H.; Qu, L. Flexible and wearable graphene/polypyrrole fibers towards multifunctional actuator applications. Electrochem. Commun. 2013, 35, 49–52. [Google Scholar] [CrossRef]
- Väntsi, O.; Kärki, T. Utilization of recycled mineral wool as filler in wood-polypropylene composites. Constr. Build. Mater. 2014, 55, 220–226. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Fu, S.Y.; Zheng, L.M.; Zhan, H.Y. Effect of nanocellulose isolation techniques on the formation of reinforced poly(vinyl alcohol) nanocomposite films. Express Polym. Lett. 2012, 6, 794–804. [Google Scholar] [CrossRef]
- Filson, P.B.; Dawson-Andoh, B.E. Sono-chemical preparation of cellulose nanocrystals from lignocellulose derived materials. Bioresour. Technol. 2009, 100, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Huang, B.; Lu, Q.; Wang, S.; Ou, W.; Lin, W.; Chen, X. Ultrasonication-assisted manufacture of cellulose nanocrystals esterified with acetic acid. Bioresour. Technol. 2013, 127, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Ago, M.; Endo, T.; Hirotsu, T. Crystalline transformation of native cellulose from cellulose I to cellulose II polymorph by a ball-milling method with a specific amount of water. Cellulose 2004, 11, 163–167. [Google Scholar] [CrossRef]
- Barakat, A.; Mayer-Laigle, C.; Solhy, A.; Arancon, R.A.D.; De Vries, H.; Luque, R. Mechanical pretreatments of lignocellulosic biomass: Towards facile and environmentally sound technologies for biofuels production. RSC Adv. 2014, 4, 48109–48127. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.; Kim, J.; Mitchell, R.J.; Lee, J.H. Environmentally friendly pretreatment of plant biomass by planetary and attrition milling. Bioresour. Technol. 2013, 144, 50–56. [Google Scholar] [CrossRef]
- Baheti, V.; Abbasi, R.; Militky, J. Ball milling of jute fibre wastes to prepare nanocellulose. World J. Eng. 2012, 9, 45–50. [Google Scholar] [CrossRef]
- Feng, Y.T.; Han, K.; Owen, D.R.J. Discrete element simulation of the dynamics of high energy planetary ball milling processes. Mater. Sci. Eng. A 2004, 375, 815–819. [Google Scholar] [CrossRef]
- Avolio, R.; Bonadies, I.; Capitani, D.; Errico, M.E.; Gentile, G.; Avella, M. A multitechnique approach to assess the effect of ball milling on cellulose. Carbohydr. Polym. 2012, 87, 265–273. [Google Scholar] [CrossRef]
- Ago, M.; Endo, T.; Okajima, K. Effect of solvent on morphological and structural change of cellulose under ball-milling. Polym. J. 2007, 39, 435–441. [Google Scholar] [CrossRef]
- Brown, E.E.; Laborie, M.P.G. Bioengineering bacterial cellulose/poly(ethylene oxide) nanocomposites. Biomacromolecules 2007, 8, 3074–3081. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Shin, E.J. The nanofication and functionalization of bacterial cellulose and its applications. Nanomaterials 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.W.; Ul-Islam, M.; Khan, S.; Kim, Y.; Park, J.K. Innovative production of bio-cellulose using a cell-free system derived from a single cell line. Carbohydr. Polym. 2015, 132, 286–294. [Google Scholar] [CrossRef]
- Crépy, L.; Chaveriat, L.; Banoub, J.; Martin, P.; Joly, N. Synthesis of Cellulose Fatty Esters as Plastics—Influence of the Degree of Substitution and the Fatty Chain Length on Mechanical Properties. ChemSusChem 2009, 2, 165–170. [Google Scholar] [CrossRef]
- Cunha, A.G.; Mougel, J.B.; Cathala, B.; Berglund, L.A.; Capron, I. Preparation of double pickering emulsions stabilized by chemically tailored nanocelluloses. Langmuir 2014, 30, 9327–9335. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaushik, A.; Ahuja, D. Surface functionalization of nanofibrillated cellulose extracted from wheat straw: Effect of process parameters. Carbohydr. Polym. 2016, 150, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Zhong, L.; Lin, X.; Chang, X.; Wu, F.; Wu, R.; Xie, F. Preparation of formyl cellulose and its enhancement effect on the mechanical and barrier properties of polylactic acid films. Int. J. Biol. Macromol. 2021, 172, 82–92. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Cao, M.; Li, J.; Wu, M.; Zhang, H.; Zheng, S.; Liu, H.; Yang, M. Combining ‘grafting to’ and ‘grafting from’ to synthesize comb-like NCC-g-PLA as a macromolecular modifying agent of PLA. Nanotechnology 2021, 32, 385601. [Google Scholar] [CrossRef]
- Hayase, G.; Kanamori, K.; Hasegawa, G.; Maeno, A.; Kaji, H.; Nakanishi, K. A superamphiphobic macroporous silicone monolith with marshmallow-like flexibility. Angew. Chem. Int. Ed. 2013, 52, 10788–10791. [Google Scholar] [CrossRef]
- Si, Y.; Guo, Z. Superhydrophobic nanocoatings: From materials to fabrications and to applications. Nanoscale 2015, 7, 5922–5946. [Google Scholar] [CrossRef] [Green Version]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Averianov, I.V.; Stepanova, M.A.; Gofman, I.V.; Nikolaeva, A.L.; Korzhikov-Vlakh, V.A.; Karttunen, M.; Korzhikova-Vlakh, E.G. Chemical modification of nanocrystalline cellulose for improved interfacial compatibility with poly(lactic acid). Mendeleev Commun. 2019, 29, 220–222. [Google Scholar] [CrossRef]
- Goussé, C.; Chanzy, H.; Cerrada, M.L.; Fleury, E. Surface silylation of cellulose microfibrils: Preparation and rheological properties. Polymer 2004, 45, 1569–1575. [Google Scholar] [CrossRef]
- Bulota, M.; Kreitsmann, K.; Hughes, M.; Paltakari, J. Acetylated microfibrillated cellulose as a toughening agent in poly(lactic acid). J. Appl. Polym. Sci. 2012, 126, E449–E458. [Google Scholar] [CrossRef]
- Navarro, J.R.G.; Bergström, L. Labelling of N-hydroxysuccinimide-modified rhodamine B on cellulose nanofibrils by the amidation reaction. RSC Adv. 2014, 4, 60757–60761. [Google Scholar] [CrossRef]
- Orelma, H.; Filpponen, I.; Johansson, L.S.; Österberg, M.; Rojas, O.J.; Laine, J. Surface functionalized nanofibrillar cellulose (NFC) film as a platform for immunoassays and diagnostics. Biointerphases 2012, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Niu, Q.; Gao, K.; Wu, W. Cellulose nanofibril based graft conjugated polymer films act as a chemosensor for nitroaromatic. Carbohydr. Polym. 2014, 110, 47–52. [Google Scholar] [CrossRef]
- Abdelmouleh, M.; Boufi, S.; Belgacem, M.N.; Dufresne, A.; Gandini, A. Modification of cellulose fibers with functionalized silanes: Effect of the fiber treatment on the mechanical performances of cellulose–thermoset composites. J. Appl. Polym. Sci. 2005, 98, 974–984. [Google Scholar] [CrossRef]
- Brochier Salon, M.C.; Abdelmouleh, M.; Boufi, S.; Belgacem, M.N.; Gandini, A. Silane adsorption onto cellulose fibers: Hydrolysis and condensation reactions. J. Colloid Interface Sci. 2005, 289, 249–261. [Google Scholar] [CrossRef]
- Van de Weyenberg, I.; Ivens, J.; De Coster, A.; Kino, B.; Baetens, E.; Verpoest, I. Influence of processing and chemical treatment of flax fibres on their composites. Compos. Sci. Technol. 2003, 63, 1241–1246. [Google Scholar] [CrossRef]
- Lu, T.; Jiang, M.; Jiang, Z.; Hui, D.; Wang, Z.; Zhou, Z. Effect of surface modification of bamboo cellulose fibers on mechanical properties of cellulose/epoxy composites. Compos. Part B Eng. 2013, 51, 28–34. [Google Scholar] [CrossRef]
- Moazzami Goudarzi, Z.; Behzad, T.; Ghasemi-Mobarakeh, L.; Kharaziha, M. An investigation into influence of acetylated cellulose nanofibers on properties of PCL/Gelatin electrospun nanofibrous scaffold for soft tissue engineering. Polymer 2021, 213, 123313. [Google Scholar] [CrossRef]
- Li, X.; Tabil, L.G.; Panigrahi, S. Chemical treatments of natural fiber for use in natural fiber-reinforced composites: A review. J. Polym. Environ. 2007, 15, 25–33. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Lucka-Gabor, M.; Gutowski, V.S. The effects of acetylation on properties of flax fibre and its polypropylene composites. Express Polym. Lett. 2008, 2, 413–422. [Google Scholar] [CrossRef]
- Venkateswarlu, M.; Rajanna, K.C.; Kumar, M.S.; Kumar, U.U.; Ramgopal, S.; Saiprakash, P.K. Rate Enhancements in the Acetylation and Benzoylation of Certain Aromatic Compounds with Vilsmeier-Haack Reagents Using Acetamide, Benzamide and Oxychlorides under Non-Conventional Conditions. Int. J. Org. Chem. 2011, 1, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Tserki, V.; Zafeiropoulos, N.E.; Simon, F.; Panayiotou, C. A study of the effect of acetylation and propionylation surface treatments on natural fibres. Compos. Part A Appl. Sci. Manuf. 2005, 36, 1110–1118. [Google Scholar] [CrossRef]
- Jamaluddin, N.; Hsu, Y.I.; Asoh, T.A.; Uyama, H. Effects of Acid-Anhydride-Modified Cellulose Nanofiber on Poly(Lactic Acid) Composite Films. Nanomaterials 2021, 11, 753. [Google Scholar] [CrossRef]
- Missoum, K.; Belgacem, M.N.; Barnes, J.P.; Brochier-Salon, M.C.; Bras, J. Nanofibrillated cellulose surface grafting in ionic liquid. Soft Matter. 2012, 8, 8338–8349. [Google Scholar] [CrossRef]
- Miki, K.; Kamitakahara, H.; Yoshinaga, A.; Tobimatsu, Y.; Takano, T. Methylation-triggered fractionation of lignocellulosic biomass to afford cellulose-, hemicellulose-, and lignin-based functional polymers via click chemistry. Green Chem. 2020, 22, 2909–2928. [Google Scholar] [CrossRef]
- Hynninen, V.; Mohammadi, P.; Wagermaier, W.; Hietala, S.; Linder, M.B.; Ikkala, O. Nonappa Methyl cellulose/cellulose nanocrystal nanocomposite fibers with high ductility. Eur. Polym. J. 2019, 112, 334–345. [Google Scholar] [CrossRef]
- Sarikaya, E.; Çallioğlu, H.; Demirel, H. Production of epoxy composites reinforced by different natural fibers and their mechanical properties. Compos. Part B Eng. 2019, 167, 461–466. [Google Scholar] [CrossRef]
- Arrakhiz, F.Z.; El Achaby, M.; Malha, M.; Bensalah, M.O.; Fassi-Fehri, O.; Bouhfid, R.; Benmoussa, K.; Qaiss, A. Mechanical and thermal properties of natural fibers reinforced polymer composites: Doum/low density polyethylene. Mater. Des. 2013, 43, 200–205. [Google Scholar] [CrossRef]
- Kim, N.K.; Lin, R.J.T.; Bhattacharyya, D. Extruded short wool fibre composites: Mechanical and fire retardant properties. Compos. Part B Eng. 2014, 67, 472–480. [Google Scholar] [CrossRef]
- Sharkawi, A.M.; Mehriz, A.M.; Showaib, E.A.; Hassanin, A. Performance of sustainable natural yarn reinforced polymer bars for construction applications. Constr. Build. Mater. 2018, 158, 359–368. [Google Scholar] [CrossRef]
- Pennafort, L.C.G.; De Queiroz, W.L.R.; De Codes, R.N.; De Deus, E.P. Caracterização mecânica do compósito PVC/fibra de coco pelos métodos de emissão acústica e de correlação digital de imagem. Cienc. Tecnol. Mater. 2014, 26, 25–32. [Google Scholar] [CrossRef]
- Khatri, J.; Patolia, H.; Brahmbhatt, K. Analysis of Mechanical Properties of Natural Fiber Composite Beam. Kalpa Publ. Eng. 2018, 1, 233–236. [Google Scholar]
- Kulachenko, A.; Denoyelle, T.; Galland, S.; Lindström, S.B. Elastic properties of cellulose nanopaper. Cellulose 2012, 19, 793–807. [Google Scholar] [CrossRef]
- De Mesquita, J.P.; Donnici, C.L.; Pereira, F.V. Biobased nanocomposites from layer-by-layer assembly of cellulose nanowhiskers with chitosan. Biomacromolecules 2010, 11, 473–480. [Google Scholar] [CrossRef]
- Jiang, C.; Tsukruk, V.V. Freestanding Nanostructures via Layer-by-Layer Assembly. Adv. Mater. 2006, 18, 829–840. [Google Scholar] [CrossRef]
- Ding, F.; Liu, J.; Zeng, S.; Xia, Y.; Wells, K.M.; Nieh, M.P.; Sun, L. Biomimetic nanocoatings with exceptional mechanical, barrier, and flame-retardant properties from large-scale one-step coassembly. Sci. Adv. 2017, 3, e170121. [Google Scholar] [CrossRef] [Green Version]
- Podsiadlo, P.; Sui, L.; Elkasabi, Y.; Burgardt, P.; Lee, J.; Miryala, A.; Kusumaatmaja, W.; Carman, M.R.; Shtein, M.; Kieffer, J.; et al. Layer-by-layer assembled films of cellulose nanowires with antireflective properties. Langmuir 2007, 23, 7901–7906. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, 411–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karabulut, E.; Wagberg, L. Design and characterization of cellulose nanofibril-based freestanding films prepared by layer-by-layer deposition technique. Soft Matter 2011, 7, 3467–3474. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, G.; Knight, D.P.; Shao, Z.; Chen, X. Wet-spinning of regenerated silk fiber from aqueous silk fibroin solution: Discussion of spinning parameters. Biomacromolecules 2010, 11, 1–5. [Google Scholar] [CrossRef]
- Lundahl, M.J.; Klar, V.; Wang, L.; Ago, M.; Rojas, O.J. Spinning of cellulose nanofibrils into filaments: A review. Ind. Eng. Chem. Res. 2017, 56, 8–19. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Q.; Wang, Y.; Liu, X.; Nie, S. Strong fibrous filaments nanocellulose crystals prepared by self-twisting microfluidic spinning. Ind. Crops Prod. 2022, 178, 114599. [Google Scholar] [CrossRef]
- He, X.; Xiao, Q.; Lu, C.; Wang, Y.; Zhang, X.; Zhao, J.; Zhang, W.; Zhang, X.; Deng, Y. Uniaxially aligned electrospun all-cellulose nanocomposite nanofibers reinforced with cellulose nanocrystals: Scaffold for tissue engineering. Biomacromolecules 2014, 15, 618–627. [Google Scholar] [CrossRef]
- Tian, L.; Luan, J.; Liu, K.K.; Jiang, Q.; Tadepalli, S.; Gupta, M.K.; Naik, R.R.; Singamaneni, S. Plasmonic Biofoam: A Versatile Optically Active Material. Nano Lett. 2016, 16, 609–616. [Google Scholar] [CrossRef]
- Nemoto, J.; Saito, T.; Isogai, A. Simple Freeze-Drying Procedure for Producing Nanocellulose Aerogel-Containing, High-Performance Air Filters. ACS Appl. Mater. Interfaces 2015, 7, 19809–19815. [Google Scholar] [CrossRef]
- Ma, G.; He, M.; Yang, G.; Ji, X.; Lucia, L.A.; Chen, J. A feasible approach efficiently redisperse dried cellulose nanofibrils in water: Vacuum or freeze drying in the presence of sodium chloride. Cellulose 2021, 28, 829–842. [Google Scholar] [CrossRef]
- Gao, H.L.; Xu, L.; Long, F.; Pan, Z.; Du, Y.X.; Lu, Y.; Ge, J.; Yu, S.H. Macroscopic Free-Standing Hierarchical 3D Architectures Assembled from Silver Nanowires by Ice Templating. Angew. Chem. Int. Ed. 2014, 53, 4561–4566. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Tsukruk, V.V. Tuning the Electronic Properties of Robust Bio-Bond Graphene Papers by Spontaneous Electrochemical Reduction: From Insulators to Flexible Semi-Metals. Chem. Mater. 2015, 27, 6717–6729. [Google Scholar] [CrossRef]
- Suzuki, S.; Shibata, Y.; Hirose, D.; Endo, T.; Ninomiya, K.; Kakuchi, R.; Takahashi, K. Cellulose triacetate synthesis via one-pot organocatalytic transesterification and delignification of pretreated bagasse. RSC Adv. 2018, 8, 21768–21776. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Tolentino, L.S.; Kulkarni, D.D.; Ye, C.; Kumar, S.; Tsukruk, V.V.; Hu, K.; Tolentino, L.S.; Kulkarni, D.D.; Ye, C.; et al. Written-in Conductive Patterns on Robust Graphene Oxide Biopaper by Electrochemical Microstamping. Angew. Chem. Int. Ed. 2013, 52, 13784–13788. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.T.T.; Zimmermann, T.; Ohr, S.; Caseri, W.R. Composites of cationic nanofibrillated cellulose and layered silicates: Water vapor barrier and mechanical properties. ACS Appl. Mater. Interfaces 2012, 4, 4832–4840. [Google Scholar] [CrossRef]
- You, Z.; Dong, Y.; Li, X.; Yang, P.; Luo, M.; Zhu, Z.; Wu, L.; Zhou, X.; Chen, M. One-pot synthesis of multi-functional cellulose-based ionic conductive organohydrogel with low-temperature strain sensitivity. Carbohydr. Polym. 2021, 251, 117019. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Xiao, S.L.; Shi, S.Q.; Cai, L.P. A one-pot synthesis and characterization of antibacterial silver nanoparticle-cellulose film. Polymers 2020, 12, 440. [Google Scholar] [CrossRef] [Green Version]
- Suntivich, R.; Drachuk, I.; Calabrese, R.; Kaplan, D.L.; Tsukruk, V.V. Inkjet printing of silk nest arrays for cell hosting. Biomacromolecules 2014, 15, 1428–1435. [Google Scholar] [CrossRef]
- Suzuki, S.; Teramoto, Y. Simple Inkjet Process to Fabricate Microstructures of Chitinous Nanocrystals for Cell Patterning. Biomacromolecules 2017, 18, 1993–1999. [Google Scholar] [CrossRef]
- Kim, J.D.; Choi, J.S.; Kim, B.S.; Chan Choi, Y.; Cho, Y.W. Piezoelectric inkjet printing of polymers: Stem cell patterning on polymer substrates. Polymer 2010, 51, 2147–2154. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Rodriguez, K.; Renneckar, S.; Vikesland, P.J. Environmental science and engineering applications of nanocellulose-based nanocomposites. Environ. Sci. Nano 2014, 1, 302–316. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Sain, M. Isolation of nanofibers from soybean source and their reinforcing capability on synthetic polymers. Compos. Sci. Technol. 2007, 67, 2521–2527. [Google Scholar] [CrossRef]
- Iwamoto, S.; Nakagaito, A.N.; Yano, H.; Nogi, M. Optically transparent composites reinforced with plant fiber-based nanofibers. Appl. Phys. A 2005, 81, 1109–1112. [Google Scholar] [CrossRef]
- Okahisa, Y.; Yoshida, A.; Miyaguchi, S.; Yano, H. Optically transparent wood–cellulose nanocomposite as a base substrate for flexible organic light-emitting diode displays. Compos. Sci. Technol. 2009, 69, 1958–1961. [Google Scholar] [CrossRef]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically Transparent Nanofiber Paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Atiqah, A.; Sabaruddin, F.A.; Ismail, N.; Jalar, A.; Bakar, M.A.; Hamzah, A.A.; Ilyas, R.A.; Asrofi, M. Nanocellulose composites for electronic applications. In Industrial Applications of Nanocellulose and Its Nanocomposites; Sapuan, S.M., Norrrahim, M.N.F., Ilyas, R.A., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 481–502. [Google Scholar]
- Sapuan, S.M.; Norrrahim, M.N.F.; Ilyas, R.A.; Soutis, C. Industrial Applications of Nanocellulose and Its Nanocomposites; Woodhead Publishing: Sawston, UK, 2022. [Google Scholar]
- Bencurova, E.; Shityakov, S.; Schaack, D.; Kaltdorf, M.; Sarukhanyan, E.; Hilgarth, A.; Rath, C.; Montenegro, S.; Roth, G.; Lopez, D.; et al. Nanocellulose Composites as Smart Devices with Chassis, Light-Directed DNA Storage, Engineered Electronic Properties, and Chip Integration. Front. Bioeng. Biotechnol. 2022, 10, 869111. [Google Scholar] [CrossRef]
- Septevani, A.A.; Burhani, D.; Sampora, Y. Nanocellulose in Electronics and Electrical Industry. In Nanocellulose Materials, Fabrication and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 217–246. [Google Scholar]
- Tammela, P.; Wang, Z.; Frykstrand, S.; Zhang, P.; Sintorn, I.M.; Nyholm, L.; Strømme, M. Asymmetric supercapacitors based on carbon nanofibre and polypyrrole/nanocellulose composite electrodes. RSC Adv. 2015, 5, 16405–16413. [Google Scholar] [CrossRef]
- Wang, Z.; Tammela, P.; Zhang, P.; Huo, J.; Ericson, F.; Strømme, M.; Nyholm, L. Freestanding nanocellulose-composite fibre reinforced 3D polypyrrole electrodes for energy storage applications. Nanoscale 2014, 6, 13068–13075. [Google Scholar] [CrossRef]
- Razaq, A.; Nyholm, L.; Sjödin, M.; Strømme, M.; Mihranyan, A. Paper-Based Energy-Storage Devices Comprising Carbon Fiber-Reinforced Polypyrrole-Cladophora Nanocellulose Composite Electrodes. Adv. Energy Mater. 2012, 2, 445–454. [Google Scholar] [CrossRef]
- Müller, D.; Cercená, R.; Gutiérrez Aguayo, A.J.; Porto, L.M.; Rambo, C.R.; Barra, G.M.O. Flexible PEDOT-nanocellulose composites produced by in situ oxidative polymerization for passive components in frequency filters. J. Mater. Sci. Mater. Electron. 2016, 27, 8062–8067. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Gumrah Dumanli, A. Nanocellulose and its Composites for Biomedical Applications. Curr. Med. Chem. 2016, 24, 512–528. [Google Scholar] [CrossRef] [PubMed]
- Guise, C.; Fangueiro, R. Biomedical applications of nanocellulose. RILEM Bookser. 2016, 12, 155–169. [Google Scholar]
- Nehra, P.; Chauhan, R.P. Eco-friendly nanocellulose and its biomedical applications: Current status and future prospect. J. Biomater. Sci. Polym. Ed. 2021, 32, 112–149. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, T.; Koivuniemi, R.; Kosonen, M.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Vuola, J.; Valtonen, J.; Tammela, P.; Mäkitie, A.; Luukko, K.; et al. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Control. Release 2016, 244, 292–301. [Google Scholar] [CrossRef]
- Hu, L.; Zheng, G.; Yao, J.; Liu, N.; Weil, B.; Eskilsson, M.; Karabulut, E.; Ruan, Z.; Fan, S.; Bloking, J.T.; et al. Transparent and conductive paper from nanocellulose fibers. Energy Environ. Sci. 2013, 6, 513–518. [Google Scholar] [CrossRef]
- Vismara, E.; Bernardi, A.; Bongio, C.; Farè, S.; Pappalardo, S.; Serafini, A.; Pollegioni, L.; Rosini, E.; Torri, G. Bacterial nanocellulose and its surface modification by glycidyl methacrylate and ethylene glycol dimethacrylate. Incorporation of vancomycin and ciprofloxacin. Nanomaterials 2019, 9, 1668. [Google Scholar] [CrossRef] [Green Version]
- Darder, M.; Karan, A.; del Real, G.; DeCoster, M.A. Cellulose-based biomaterials integrated with copper-cystine hybrid structures as catalysts for nitric oxide generation. Mater. Sci. Eng. C 2020, 108, 110369. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Hosseinidoust, Z.; Alam, M.N.; Sim, G.; Tufenkji, N.; Van De Ven, T.G.M. Cellulose nanocrystals with tunable surface charge for nanomedicine. Nanoscale 2015, 7, 16647–16657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, J.; Cui, Z.; Wang, Q.; Liu, Q.; Liu, C. Biomimetic composite scaffolds based on mineralization of hydroxyapatite on electrospun poly(ɛ-caprolactone)/nanocellulose fibers. Carbohydr. Polym. 2016, 143, 270–278. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef] [Green Version]
- Sampath, U.G.T.M.; Ching, Y.C.; Chuah, C.H.; Singh, R.; Lin, P.C. Preparation and characterization of nanocellulose reinforced semi-interpenetrating polymer network of chitosan hydrogel. Cellulose 2017, 24, 2215–2228. [Google Scholar] [CrossRef]
- Yang, H.; Tejado, A.; Alam, N.; Antal, M.; Ven, T.G.M. Van De Films prepared from electrosterically stabilized nanocrystalline cellulose. Langmuir 2012, 28, 7834–7842. [Google Scholar] [CrossRef] [PubMed]
- Ahankari, S.S.; Subhedar, A.R.; Bhadauria, S.S.; Dufresne, A. Nanocellulose in food packaging: A review. Carbohydr. Polym. 2021, 255, 117479. [Google Scholar] [CrossRef]
- Vilarinho, F.; Sanches Silva, A.; Vaz, M.F.; Farinha, J.P. Nanocellulose in green food packaging. Crit. Rev. Food Sci. Nutr. 2018, 58, 1526–1537. [Google Scholar] [CrossRef]
- Trifol, J.; Plackett, D.; Sillard, C.; Szabo, P.; Bras, J.; Daugaard, A.E. Hybrid poly(lactic acid)/nanocellulose/nanoclay composites with synergistically enhanced barrier properties and improved thermomechanical resistance. Polym. Int. 2016, 65, 988–995. [Google Scholar] [CrossRef] [Green Version]
- Silva, F.A.G.S.; Dourado, F.; Gama, M.; Poças, F. Nanocellulose Bio-Based Composites for Food Packaging. Nanomaterials 2020, 10, 2041. [Google Scholar] [CrossRef]
- Nair, S.S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. High performance green barriers based on nanocellulose. Sustain. Chem. Process. 2014, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose-based materials for water purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.H.; Omer, A.M.; Ouyang, X.K.; Yu, D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Jebali, A.; Behzadi, A.; Rezapor, I.; Jasemizad, T.; Hekmatimoghaddam, S.H.; Halvani, G.H.; Sedighi, N. Adsorption of humic acid by amine-modified nanocellulose: An experimental and simulation study. Int. J. Environ. Sci. Technol. 2015, 12, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Hakami, M.W.; Alkhudhiri, A.; Zacharof, M.P.; Hilal, N. Towards a Sustainable Water Supply: Humic Acid Removal Employing Coagulation and Tangential Cross Flow Microfiltration. Water 2019, 11, 2093. [Google Scholar] [CrossRef]
- Ge, L.; Yin, J.; Yan, D.; Hong, W.; Jiao, T. Construction of Nanocrystalline Cellulose-Based Composite Fiber Films with Excellent Porosity Performances via an Electrospinning Strategy. ACS Omega 2021, 6, 4958–4967. [Google Scholar] [CrossRef]
- Tyagi, P.; Hubbe, M.A.; Lucia, L.; Pal, L. High performance nanocellulose-based composite coatings for oil and grease resistance. Cellulose 2018, 25, 3377–3391. [Google Scholar] [CrossRef]
- Zimmermann, M.V.G.; da Silva, M.P.; Zattera, A.J.; Campomanes Santana, R.M. Effect of nanocellulose fibers and acetylated nanocellulose fibers on properties of poly(ethylene-co-vinyl acetate) foams. J. Appl. Polym. Sci. 2017, 134, 44760. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Yano, H. The effect of fiber content on the mechanical and thermal expansion properties of biocomposites based on microfibrillated cellulose. Cellulose 2008, 15, 555–559. [Google Scholar] [CrossRef]
- Zheng, X.; Fu, S. Reconstructing micro/nano hierarchical structures particle with nanocellulose for superhydrophobic coatings. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 171–179. [Google Scholar] [CrossRef]
- Cherian, R.M.; Tharayil, A.; Varghese, R.T.; Antony, T.; Kargarzadeh, H.; Chirayil, C.J.; Thomas, S. A review on the emerging applications of nano-cellulose as advanced coatings. Carbohydr. Polym. 2022, 282, 119123. [Google Scholar] [CrossRef]
- Makarona, E.; Koutzagioti, C.; Salmas, C.; Ntalos, G.; Skoulikidou, M.C.; Tsamis, C. Enhancing wood resistance to humidity with nanostructured ZnO coatings. Nano-Struct. Nano-Objects 2017, 10, 57–68. [Google Scholar] [CrossRef]
- Cataldi, A.; Esposito Corcione, C.; Frigione, M.; Pegoretti, A. Photocurable resin/nanocellulose composite coatings for wood protection. Prog. Org. Coat. 2017, 106, 128–136. [Google Scholar] [CrossRef]










| Nanocellulose Types | Sources | Extraction Method and Size |
|---|---|---|
| CNC | Cotton, tunicin, mulberry bark, hemp, wood, wheat straw. | Acid hydrolysis 5–70 nm in diameter 100–250 nm in length |
| BNC | Sugars and alcohols. | Extracted from bacterial synthesis 20–100 nm in diameter |
| NFC | Wood, hemp, flax, potato tuber, sugar beet. | A mechanical method of breaking the cellulose 5–60 nm in diameter |
| Biological Methods | Mechanical Methods | Chemical Methods |
|---|---|---|
| Fungi treatment | Steam explosion | Ionic treatment |
| Bacteria treatment | Ball milling | Alkaline treatment |
| Enzymatic hydrolysis | Disintegration | Acid hydrolysis |
| Grinding | Oxidation | |
| Electrospinning | Solvent extraction | |
| Ultrasonication | ||
| Homogenization |
| Modification Method | Chemical Sources | Modified Characteristics | References |
|---|---|---|---|
| Silylation | Alkoxy silane, triethoxyvinylsilane, chlorodimethyl isopropylsilane | Hydrocarbon chains in silane enhance the wettability of cellulose. | [65] |
| Esterification | Aromatic and aliphatic carboxylic reagents (acidic anhydride) | Plasticization of lignocellulosic strands due to interaction of OH groups of cellulose with acetyl moieties. | [66] |
| Carbamylation | Isocynaic acids (Butyl 4-(Boc-aminomethyl) phenyl isothiocyanate) | Bonding of functional groups of cellulose with isocyanic acid. | [67] |
| Functionalized reactions | TEMPO oxidizers (sodium hypochlorites) | Attachment of carboxyl groups on the cellulose surface to initiate further reactions. | [68,69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir, D.; Karim, M.R.A.; Hu, H.; Naseem, S.; Rehan, M.; Ahmad, M.; Zhang, M. Sources, Chemical Functionalization, and Commercial Applications of Nanocellulose and Nanocellulose-Based Composites: A Review. Polymers 2022, 14, 4468. https://doi.org/10.3390/polym14214468
Tahir D, Karim MRA, Hu H, Naseem S, Rehan M, Ahmad M, Zhang M. Sources, Chemical Functionalization, and Commercial Applications of Nanocellulose and Nanocellulose-Based Composites: A Review. Polymers. 2022; 14(21):4468. https://doi.org/10.3390/polym14214468
Chicago/Turabian StyleTahir, Danish, Muhammad Ramzan Abdul Karim, Hong Hu, Sufyan Naseem, Muhammad Rehan, Mairaj Ahmad, and Minglonghai Zhang. 2022. "Sources, Chemical Functionalization, and Commercial Applications of Nanocellulose and Nanocellulose-Based Composites: A Review" Polymers 14, no. 21: 4468. https://doi.org/10.3390/polym14214468
APA StyleTahir, D., Karim, M. R. A., Hu, H., Naseem, S., Rehan, M., Ahmad, M., & Zhang, M. (2022). Sources, Chemical Functionalization, and Commercial Applications of Nanocellulose and Nanocellulose-Based Composites: A Review. Polymers, 14(21), 4468. https://doi.org/10.3390/polym14214468