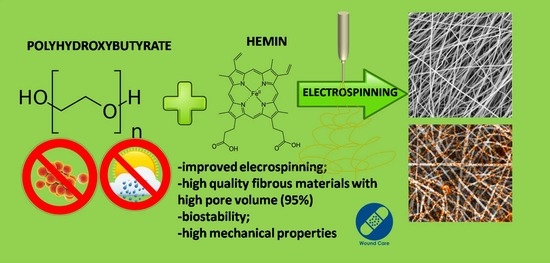
Bioinspired Electropun Fibrous Materials Based on Poly-3-Hydroxybutyrate and Hemin: Preparation, Physicochemical Properties, and Weathering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrospun Materials
2.3. Methods
2.3.1. Optical Microscopy
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Estimation of Structural Parameters
2.3.4. Energy-Dispersive X-ray Spectroscopy (EDX)
2.3.5. FTIR Spectroscopy
2.3.6. Differential Scanning Calorimetry (DSC)
2.3.7. Electronic Paramagnetic Resonance (EPR)
2.3.8. Mechanical Tests
2.3.9. Weathering Experiments upon Outdoor Exposure
2.3.10. Statistical Analysis
3. Results
3.1. Morphology Structure and Properties of Hemin/PHB Fibrous Materials
3.2. Outdoor Exposure of Hemin/PHB Fibrous Materials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Poltronieri, P.; Kumar, P. Polyhydroxyalcanoates (PHAs) in Industrial Applications. In Handbook of Ecomaterials; Springer: Cham, Switzerland, 2017; pp. 1–30. [Google Scholar] [CrossRef]
- Rajan, K.P.; Thomas, S.P.; Gopanna, A.; Chavali, M. Polyhydroxybutyrate (PHB): A Standout Biopolymer for Environmental Sustainability. In Handbook of Ecomaterials; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 1–23. [Google Scholar] [CrossRef]
- Kostopoulos, V.; Kotrotsos, A.; Fouriki, K.; Kalarakis, A.; Portan, D. Fabrication and Characterization of Polyetherimide Electrospun Scaffolds Modified with Graphene Nano-Platelets and Hydroxyapatite Nano-Particles. Int. J. Mol. Sci. 2020, 21, 583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dror, Y.; Salalha, W.; Khalfin, R.L.; Cohen, Y.; Yarin, A.L.; Zussman, E. Carbon nanotubes embeded in oriented polymer nanofibers by electrospinning. Langmuir 2003, 19, 7012–7020. [Google Scholar] [CrossRef]
- Moydeen, M.A.; Syed, A.M.; Padusha, E.F.; Aboelfetoh, S.S.; Al-Deyab, M.H. Fabrication of electrospun poly(vinyl alcohol)/dextran nanofibers via emulsion process as drug delivery system: Kinetics and in vitro release study. Int. J. of Biol. Macromol. 2018, 116, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.; van der Velden, N.; Persson, N.-K.; Hamedi, M.; Müller, C. Electrically conducting fibres for e-textiles: An open playground for conjugated polymers and carbon nanomaterials. Mater. Sci. Eng. R Rep. 2018, 126, 1–29. [Google Scholar] [CrossRef]
- Bailey, F.; Malinski, T.; Kiechle, F. Carbon-fiber ultramicroelectrodes modified with conductive polymeric tetrakis(3-methoxy-4-hydroxyphenyl)porphyrin for determination of nickel in single biological cells. Anal. Chem. 1991, 63, 395–398. [Google Scholar] [CrossRef]
- Avossa, J.; Paolesse, R.; Di Natale, C.; Zampetti, E.; Bertoni, G.; De Cesare, F.; Scarascia-Mugnozza, G.; Macagnano, A. Electrospinning of polystyrene/polyhydroxybutyrate nanofibers doped with porphyrin and graphene for chemiresistor gas sensors. Polymers 2019, 9, 280. [Google Scholar] [CrossRef] [Green Version]
- Tabassum, R.; Kant, R. Recent trends in surface plasmon resonance based fiber–optic gas sensors utilizing metal oxides and carbon nanomaterials as functional entities. Sens. Actuators B Chem. 2020, 310, 127813. [Google Scholar] [CrossRef]
- Scheicher, S.R.; Kainz, B.; Köstler, S.; Suppan, M.; Bizzarri, A.; Pum, D.; Sleytr, U.; Ribitsch, V. Optical oxygen sensors based on Pt(II) porphyrin dye immobilized on S-layer protein matrices. Biosens. Bioelectron. 2009, 25, 797–802. [Google Scholar] [CrossRef]
- Laskowska, M.; Kityk, L.; Pastukh, O.; Dulski, M.; Zubko, M.; Jedryka, J.; Laskowski, E. Nanocomposite for photonics—Nickel pyrophosphate nanocrystals synthesised in silica nanoreactors. Microporous Mesoporous Mater. 2020, 306, 110435. [Google Scholar] [CrossRef]
- Biswas, S.; Ahn, H.-Y.; Bondar, M.V.; Belfield, K.D. Two-photon absorption enhancement of polymer-templated porphyrin-based J-aggregates. Langmuir 2012, 28, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mai, B.; Tan, H.; Chen, X. The effect of thermally developed SiC@SiO2 core-shell structured nanoparticles on the mechanical, thermal and UV-shielding properties of polyimide composites. Compos. Commun. 2018, 10, 194–204. [Google Scholar] [CrossRef]
- Suo, Z.; Chen, J.; Hou, X.; Hu, Z.; Xing, F.; Feng, L. Growing prospects of DNA nanomaterials in novel biomedical applications. RSC Adv. 2019, 9, 16479–16491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosal, K.; Agatemor, C.; Špitálsky, Z.; Thomas, S.; Kny, E. Electrospinning tissue engineering and wound dressing scaffolds from polymer–titanium dioxide nanocomposites. Chem. Eng. J. 2018, 358, 1262–1278. [Google Scholar] [CrossRef]
- Wu, J.; Li, S.; Wei, H. Integrated nanozymes: Facile preparation and biomedical applications. Chem. Commun. 2018, 54, 6520–6530. [Google Scholar] [CrossRef]
- Ruthard, C.; ScHmidt, M.; Gröhn, F. Porphyrin-polymer networks, worms, and nanorods: pH-triggerable hierarchical self-assembly. Macromol. Rapid. Commun. 2011, 32, 706–711. [Google Scholar] [CrossRef]
- Sessler, J.L.; Tomat, E. Transition-metal complexes of expanded porphyrins. Acc. Chem. Res. 2007, 40, 371–379. [Google Scholar] [CrossRef]
- Sessler, J.; Miller, R. Texaphyrins: New drugs with diverse clinical applications in radiation and photodynamic therapy. Biochem. Pharmacol. 2000, 59, 733–739. [Google Scholar] [CrossRef]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as diagnostic and therapeutic agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef]
- Habermeyer, B.; Guilard, R. Some activities of Porphy Chem illustrated by the applications of porphyrinoids in PDT, PIT and PDI. Photochem. Photobiol. Sci. 2018, 17, 1675–1690. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, R.; Yu, H.; Xu, Z.; Kang, Y.; Cui, H.; Xue, P. MnO2-capped silk fibroin (SF) nanoparticles with chlorin e6 (Ce6) encapsulation for augmented photo-driven therapy by modulating the tumor microenvironment. J. Mater. Chem. B 2021, 9, 3677–3688. [Google Scholar] [CrossRef] [PubMed]
- Alsharabasy, A.M.; Pandit, A.; Farràs, P. Recent advances in the design and sensing applications of hemin/coordination polymer-based nanocomposites. Adv. Mater. 2020, 33, 2003883. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, D.; Watanabe, T.F.; Eady, R.R.; Garratt, R.C.; Antonyuk, S.V.; Hasnain, S.S. Reverse protein engineering of a novel 4-domain copper nitrite reductase reveals functional regulation by protein-protein interaction. FEBS J. 2021, 288, 262–280. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Li, B. Self-assembly of hemin on carbon nanotube as highly active peroxidase mimetic and its application for biosensing. RSC Adv. 2013, 3, 6044. [Google Scholar] [CrossRef]
- Dell’Acqua, S.; Massardi, E.; Monzani, E.; Di Natale, G.; Rizzarelli, E.; Casella, L. Interaction between hemin and prion peptides: Binding, oxidative reactivity and aggregation. Int. J. Mol. Sci. 2020, 21, 7553. [Google Scholar] [CrossRef] [PubMed]
- Zozulia, O.; Korendovych, I.V. Semi-rationally designed short peptides self-assemble and bind hemin to promote cyclopropanation. Angew. Chem. Int. Ed. 2020, 59, 8108–8112. [Google Scholar] [CrossRef]
- Qu, R.; Shen, L.; Chai, Z.; Jing, C.; Zhang, Y.; An, Y.; Shi, L. Hemin-block copolymer micelle as an artificial peroxidase and its applications in chromogenic detection and biocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 19207–19216. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Chen, G.; Li, G. Regulation of thrombin activity with a bifunctional aptamer and hemin: Development of a new anticoagulant and antidote pair. Chembiochem 2009, 10, 2171–2176. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Wei, W.; Li, Y.; Liu, A.; Zhang, Y.; Liu, S. Effect of annealing temperature and element composition of titanium dioxide/graphene/hemin catalysts for oxygen reduction reaction. RSC Adv. 2015, 5, 82879–82886. [Google Scholar] [CrossRef]
- Nitzan, Y.; Ladan, H.; Gozansky, S.; Malik, Z. Characterization of hemin antibacterial action on Staphylococcus aureus. FEMS Microbiol. Lett. 1987, 48, 401–406. [Google Scholar] [CrossRef]
- Sedaghat, S.; Shamspur, T.; Mohamadi, M.; Mostafavi, A. Extraction and preconcentration of hemin from human blood serum and breast cancer supernatant. J. Sep. Sci. 2015, 38, 4286–4291. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Song, L.; Zhang, X.; Zhou, R.; Yin, J.; Luan, S. Poly(γ-glutamic acid)-based electrospun nanofibrous mats with photodynamic therapy for effectively combating wound infection. Mater. Sci. Eng. C 2020, 113, 110936. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Sun, X.; Zhou, L.; Du, H.; Zhu, Z.; Wen, Y. Electrospun pullulan/PVA nanofibers integrated with thymol-loaded porphyrin metal-organic framework for antibacterial food packaging. Carbohydr. Polym. 2021, 270, 118391. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, C.M.A.; Iudici, M.; Spitaleri, L.; Randazzo, R.; Gaeta, M.; D’Urso, A.; Fragalà, M.E. Polyethersulfone mats function-alized with porphyrin for removal of para-nitroaniline from aqueous solution. Molecules 2019, 24, 3344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, M.S.; Fangueiro, R.; Ferreira, P.D. Drug Delivery Systems for Photodynamic Therapy: The Potentiality and Versatility of Electrospun Nanofibers. Macromol. Biosci. 2022, 22, e2100512. [Google Scholar] [CrossRef]
- Lv, D.; Wang, R.; Tang, G.; Mou, Z.; Lei, J.; Han, J.; Huang, C. Eco-friendly Electrospun Membranes Loaded with Visible-light Response Nano-particles for Multifunctional usages: High-efficient Air Filtration, Dye Scavenger and Bactericide. ACS Appl. Mater. Interfaces 2019, 11, 12880–12889. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Cui, J.; Qu, Q.; Wang, Y.; Zhang, J.; Xiong, R.; Huang, C. Multistructured Electrospun Nanofibers for Air Filtration: A Review. ACS Appl. Mater. Interfaces 2021, 13, 23293–23313. [Google Scholar] [CrossRef]
- Nakayama, D.; Wu, F.; Mohanty, A.K.; Hirai, S.; Misra, M. Biodegradable Composites Developed from PBAT/PLA Binary Blends and Silk Powder: Compatibilization and Performance Evaluation. ACS Omega 2018, 3, 12412–12421. [Google Scholar] [CrossRef] [Green Version]
- Nofar, M.; Salehiyan, R.; Ciftci, U.; Jalali, A.; Durmuş, A. Ductility improvements of PLA-based binary and ternary blends with controlled morphology using PBAT, PBSA, and nanoclay. Compos. B. Eng. 2019, 182, 107661. [Google Scholar] [CrossRef]
- Huang, M.-H.; Li, S.; Hutmacher, D.W.; Coudane, J.; Vert, M. Degradation characteristics of poly(ε-caprolactone)-based co-polymers and blends. J. Appl. Polym. Sci. 2016, 102, 1681–1687. [Google Scholar] [CrossRef]
- Dehant, I. Infrared Spectroscopy Of Polymers; Chemistry: Moscow, Russia, 1976. [Google Scholar]
- Tarasevich, B.N. IR spectra of general classes of organic compounds. In Reference Material; Publishing House of MSU: Moscow, Russia, 2012. [Google Scholar]
- Bellamy, L. The Infra-Red Spectra of Complex Molecules; Springer: Amsterdam, The Netherlands, 1975. [Google Scholar]
- Vyazovkin, S.; Koga, N.; Schick, C. Handbook of Thermal Analysis and Calorimetry, Applications to Polymers and Plastics; Elsevier: London, UK, 2002. [Google Scholar]
- Zhu, G.; Kremenakova, D.; Wang, Y.; Militky, J. Air permeability of polyester nonwoven fabrics. Autex Res. J. 2015, 15, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Scandola, M.; Focarete, M.L.; Adamus, G.; Sikorska, W.; Baranowska, I.; Świerczek, S.; Gnatowski, M.; Kowalczuk, M.; Jedliński, Z. Polymer blends of natural poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and a synthetic atactic poly(3-hydroxybutyrate). Characterization and biodegradation studies. Macromolecules 1997, 30, 2568–2574. [Google Scholar] [CrossRef]
- Sezer, D.; Freed, J.H.; Roux, B. Simulating electron spin resonance spectra of nitroxide spin labels from molecular dynamics and stochastic trajectories. J. Chem. Phys. 2008, 128, 165106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domaschke, S.; Zündel, M.; Mazza, E.; Ehret, A.E. A 3D computational model of electrospun networks and its application to inform a reduced modelling approach. Int. J. Solids Struct. 2019, 178, 76–89. [Google Scholar] [CrossRef]
- Katsogiannis, K.A.G.; Vladisavljević, G.T.; Georgiadou, S. Porous electrospun polycaprolactone (PCL) fibres by phase separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar] [CrossRef] [Green Version]
- Reneker, D.H.; Yarian, A.L.; Zussman, E.; Xu, H. Electrospinning of nanofibers from polymer solutions and melts. Adv. Appl. Mech. 2007, 41, 43–195. [Google Scholar]
- Ding, G.; Liu, J. Morphological varieties and kinetic behaviors of poly(3-hydroxybutyrate) (PHB) spherulites crystallized isothermally from thin melt film. Colloid Polym. Sci. 2013, 291, 1547–1554. [Google Scholar] [CrossRef]
- Zhao, L.; Qu, R.; Li, A.; Ma, R.; Shi, L. Cooperative self-assembly of porphyrins with polymers possessing bio-active functions. Chem. Comm. 2016, 52, 13543–13555. [Google Scholar] [CrossRef]
- Tyubaeva, P.; Varyan, I.; Krivandin, A.; Shatalova, O.; Karpova, S.; Lobanov, A.; Olkhov, A.; Popov, A. The Comparison of Advanced Electrospun Materials Based on Poly(-3-hydroxybutyrate) with Natural and Synthetic Additives. J. Funct. Biomater. 2022, 13, 23. [Google Scholar] [CrossRef]
- Arai, T.; Tanaka, M.; Kawakami, H. Porphyrin-containing electrospun nanofibers: Positional control of porphyrin molecules in nanofibers and their catalytic application. ACS Appl. Mater. Interfaces 2012, 4, 5453–5457. [Google Scholar] [CrossRef]
- Syerko, E.; Comas-Cardona, S.; Binetruy, C. Models of mechanical properties/behavior of dry fibrous materials at various scales in bending and tension: A review. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1365–1388. [Google Scholar] [CrossRef]
- Baumgarten, P.K. Electrostatic spinning of acrylic microfibers. J. Colloid Interface Sci. 1971, 36, 71–79. [Google Scholar] [CrossRef]
- Allais, M.; Mailley, D.; Hébraud, P.; Ihiawakrim, D.; Ball, V.; Meyer, F.; Hébraud, A.; Schlatter, G. Polymer-free electrospinning of tannic acid and cross-linking in water for hybrid supramolecular nanofibres. Nanoscale 2018, 10, 9164–9173. [Google Scholar] [CrossRef]
- Hannay, N.B. Treatise on Solid State Chemistry, Crystalline and Noncrystalline Solids; Plenum Press: New York, NY, USA, 1976. [Google Scholar] [CrossRef]
- Kumar, S.; Pal, S.; Ray, S. Study of microbes having potentiality for biodegradation of plastics. Environ. Sci. Pollut. Res. 2013, 20, 4339–4355. [Google Scholar] [CrossRef]
- Boopathy, R. Factors limiting bioremediation technologies. Bioresour. Technol. 2000, 74, 63–67. [Google Scholar] [CrossRef]
- Wang, Z. Biodegradation of polyhydroxybutyrate film by Pseudomonas mendocinaDS04-T. Polym. Plast. Technol. Eng. 2013, 52, 195–199. [Google Scholar] [CrossRef]
- Bonartseva, G.; Myshkina, V.; Nikolaeva, D.; Kevbrina, M.; Kallistova, A.; Gerasin, V.; Iordanskii, A.; Nozhevnikova, A. Aerobic and anaerobic microbial degradation of poly-β-hydroxybutyrate produced by Azotobacter chroococcum. Appl. Biochem. Biotechnol. 2003, 109, 285–301. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Biodegradation of different formulations of polyhydroxybutyrate films in soil. Springer Plus 2016, 5, 762. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.; Ugwu, C.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [Green Version]
- Tyubaeva, P.; Zykova, A.; Podmasteriev, V.; Olkhov, A.; Popov, A.; Iordanskii, A. The investigation of the structure and properties of ozone-sterilized nonwoven biopolymer materials for medical applications. Polymers 2021, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Mosnáčková, K.; Danko, M.; Šišková, A.; Falco, L.; Janigová, I.; Chmela, Š.; Vanovčanová, Z.; Omaníková, L.; Chodáka, I.; Mosnáček, J. Complex study of the physical properties of a poly(lactic acid)/poly(3-hydroxybutyrate) blend and its carbon black composite during various outdoor and laboratory ageing conditions. RSC Adv. 2017, 7, 47132–47142. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chou, I.-N.; Tsai, Y.; Wu, H.-S. Thermal degradation of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in drying treatment. J. Appl. Polym. Sci. 2013, 130, 3659–3667. [Google Scholar] [CrossRef]
- Haslböck, M.; Klotz, M.; Steiner, L.; Sperl, J.; Sieber, V.; Zollfrank, C.; Van Opdenbosch, D. Structures of mixed-tacticity polyhydroxybutyrates. Macromolecules 2018, 51, 5001–5010. [Google Scholar] [CrossRef]
- Ladan, H.; Nitzan, Y.; Malik, Z. The antibacterial activity of haemin compared with cobalt, zinc and magnesium protoporphyrin and its effect on pottassium loss and ultrastructure of Staphylococcus aureus. FEMS Microbiol. Lett. 1993, 112, 173–177. [Google Scholar] [CrossRef]
- Stojiljkovic, I.; Evavold, B.D.; Kumar, V. Antimicrobial properties of porphyrins. Expert Opin. Investig. Drugs 2001, 10, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Le Guern, F.; Ouk, T.S.; Yerzhan, I.; Nurlykyz, Y.; Arnoux, P.; Frochot, C.; Leroy-Lhez, S.; Sol, V. Photophysical and Bactericidal Properties of Pyridinium and Imidazolium Porphyrins for Photodynamic Antimicrobial Chemotherapy. Molecules 2021, 26, 1122. [Google Scholar] [CrossRef] [PubMed]











| Content of Hmi % | Density (Mean ± SD, n = 10) g/cm3 | Average Diameter of Fibers (Mean ± SD, n = 100) µm | Porosity (Mean ± SD, n = 10) % |
|---|---|---|---|
| 0 | 0.30 ± 0.01 | 3.50 ± 0.08 | 80 ± 2.0 |
| 1 | 0.20 ± 0.02 | 2.06 ± 0.07 | 92 ± 1.5 |
| 3 | 0.20 ± 0.01 | 1.77 ± 0.04 | 92 ± 1.5 |
| 5 | 0.17 ± 0.01 | 1.77 ± 0.04 | 94 ± 1.2 |
| Content of Hmi % | Tensile Strength (Mean ± SD = 0.05, n = 10) MPa | Elongation at Break (Mean ± SD = 0.2, n = 10) % |
|---|---|---|
| 0 | 1.7 | 3.6 |
| 1 | 0.7 | 4.7 |
| 3 | 1.9 | 4.7 |
| 5 | 5.5 | 6.1 |
| Sample | Exposure Time Days | First Heating Run | Second Heating Run | ||
|---|---|---|---|---|---|
| Tm, °C | ΔH, J/g | Tm, °C | ΔH, J/g | ||
| Hmi/PHB (5 wt.%) | 0 | 174.5 | 88.7 | 171.0 | 87.9 |
| Hmi/PHB (5 wt.%) | 90 | 171.3 | 82.8 | 166.9 | 80.2 |
| PHB | 0 | 174.1 | 73.6 | 170.4 | 79.4 |
| PHB | 90 | 170.8 | 63.2 | 167.2 | 64.9 |
| Content of Hmi % | Exposure Time Days | Tensile Strength (Mean ± SD = 0.05, n = 10) MPa | Elongation at Break (Mean ± SD = 0.2, n = 10) % |
|---|---|---|---|
| 5 | 0 | 5.5 | 6.1 |
| 5 | 90 | 1.9 | 4.5 |
| 0 | 0 | 1.7 | 3.6 |
| 0 | 90 | 2.7 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyubaeva, P.M.; Varyan, I.A.; Zykova, A.K.; Yarysheva, A.Y.; Ivchenko, P.V.; Olkhov, A.A.; Arzhakova, O.V. Bioinspired Electropun Fibrous Materials Based on Poly-3-Hydroxybutyrate and Hemin: Preparation, Physicochemical Properties, and Weathering. Polymers 2022, 14, 4878. https://doi.org/10.3390/polym14224878
Tyubaeva PM, Varyan IA, Zykova AK, Yarysheva AY, Ivchenko PV, Olkhov AA, Arzhakova OV. Bioinspired Electropun Fibrous Materials Based on Poly-3-Hydroxybutyrate and Hemin: Preparation, Physicochemical Properties, and Weathering. Polymers. 2022; 14(22):4878. https://doi.org/10.3390/polym14224878
Chicago/Turabian StyleTyubaeva, Polina M., Ivetta A. Varyan, Anna K. Zykova, Alena Yu. Yarysheva, Pavel V. Ivchenko, Anatoly A. Olkhov, and Olga V. Arzhakova. 2022. "Bioinspired Electropun Fibrous Materials Based on Poly-3-Hydroxybutyrate and Hemin: Preparation, Physicochemical Properties, and Weathering" Polymers 14, no. 22: 4878. https://doi.org/10.3390/polym14224878
APA StyleTyubaeva, P. M., Varyan, I. A., Zykova, A. K., Yarysheva, A. Y., Ivchenko, P. V., Olkhov, A. A., & Arzhakova, O. V. (2022). Bioinspired Electropun Fibrous Materials Based on Poly-3-Hydroxybutyrate and Hemin: Preparation, Physicochemical Properties, and Weathering. Polymers, 14(22), 4878. https://doi.org/10.3390/polym14224878