Development of Water Repellent, Non-Friable Tannin-Furanic-Fatty Acids Biofoams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
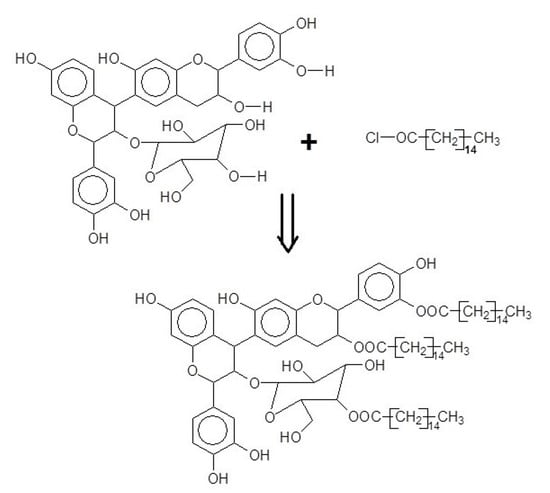
2.2. Synthesis of Oil-Grafted Tannin
2.3. Testing and Characterization of Oil-Grafted Tannin Extract
2.3.1. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
2.3.2. Matrix-Assisted Laser Desorption Ionization–Time-of-Flight (MALDI-TOF) Analysis
2.4. Foam Preparation
2.5. Characterization of Tannin-Based Foam
2.5.1. Density
2.5.2. Friability
2.5.3. Contact Angle Measurement
3. Results and Discussion
3.1. FTIR Results of Oil-Grafted Tannin
3.2. MALDI-TOF Analysis
3.2.1. MALDI-TOF Analysis of Oil-Grafted Tannins from Palmitoyl Chloride
3.2.2. MALDI-TOF Analysis of Oil-Grafted Tannins from Oleoyl Chloride
3.2.3. MALDI-TOF Analysis of Oil-Grafted Tannins from Lauryl Chloride
3.3. Density and Friability
3.4. Wettability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Pizzi, A. Tannin-based Adhesives. In Wood adhesives Chemistry and Technology; Marcel Dekker: New York, NY, USA, 1983; pp. 177–246. [Google Scholar]
- Pizzi, A. Advanced Wood Adhesives Technology; Marcel Dekker: New York, NY, USA, 1994; pp. 149–217. [Google Scholar]
- Valenzuela, J.; von Leyser, E.; Pizzi, A.; Westermeyer, C.; Gorrini, B. Industrial production of pine tannin-bonded particleboard and MDF. Eur. J. Wood Prod. 2012, 70, 735–740. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Prospectives and actual industrial applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzi, A. Tannins medical/pharmacological and related applications: A critical review. Sustain. Chem. Pharm. 2021, 22, 100481. [Google Scholar] [CrossRef]
- Meikleham, N.; Pizzi, A. Acid and alkali-setting tannin-based rigid foams. J. Appl. Polym. Sci. 1994, 53, 1547–1556. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-based Biofoams. J. Renew. Mater. 2019, 7, 477–492. [Google Scholar] [CrossRef] [Green Version]
- Merle, J.; Sénéchal, P.; Guerton, F.; Moonen, P.; Trinsoutrot, P.; Birot, M.; Charrier-El Bouhtoury, F. Microstructural characterization of biobased carbon foam by means of X-ray microtomography and compared to conventional techniques. ACS Adv. 2016, 6, 96057–96064. [Google Scholar] [CrossRef]
- Khundamri, N.; Aouf, C.; Fulcrand, H.; Dubreucq, E.; Tanrattanakul, V. Bio-based flexible epoxy foam synthesized from epoxidized soybean oil and epoxidized mangosteen tannin. Ind. Crops Prod. 2019, 128, 556–565. [Google Scholar] [CrossRef]
- Sahimim, W.; Boer, F.D.; Chapuis, H.; Obonou-Akong, F.; Pizzi, A.; Gerardin, P.; Gerardin-Charbonnier, C. Feasibility Study of the Synthesis of Isocyanate-Free Polyurethanes from Catechin. J. Renew. Mater. 2022, 10, 1175–1184. [Google Scholar] [CrossRef]
- Azadeh, E.; Chen, X.; Pizzi, A.; Gérardin, C.; Gérardin, P.; Essawy, H. Self-Blowing Non-Isocyanate Polyurethane Foams Based on Hydrolysable Tannins. J. Renew. Mater. 2022, 10, 3217–3227. [Google Scholar] [CrossRef]
- Shirmohamadi, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a Sustainable Raw Material for Green Chemistry: A review. Ind. Crops Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Zhao, W.; Pizzi, A.; Fierro, V.; Du, G.; Celzard, A. Effect of composition and processing parameters on the characteristics of tannin-based rigid foams. Part 1: Cell structure. Mater. Chem. Phys. 2010, 122, 175–182. [Google Scholar] [CrossRef]
- Cop, M.; Lacoste, C.; Conradi, M.; Laborie, M.-P.; Pizzi, A.; Sernek, M. The effect of the composition of spruce and pine tannin-based foams on their physical, morphological and compression properties. Ind. Crops Prod. 2015, 74, 158–164. [Google Scholar] [CrossRef]
- Böhm, R.; Hauptmann, M.; Pizzi, A.; Friederich, C.; Laborie, M.-P. The chemical, kinetic and mechanical characterization of Tannin-based adhesives with different crosslinking systems. Int. J. Adhesion Adhes. 2016, 68, 1–8. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Lei, H.; Du, G.; Zhou, X.; Lin, Y. Characterization and preparation of furanic-glyoxal foams. Polymers 2020, 12, 692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Li, J.; Xi, X.; Pizzi, A.; Zhou, X.; Fredon, E.; Du, G.; Gerardin, C. Condensed Glucose-Tannin-based NIPU BioFoams of Improved Fire Retardancy. Polymer Degr. Stabil. 2020, 17, 109121. [Google Scholar] [CrossRef]
- Basso, M.C.; Giovando, S.; Pizzi, A.; Celzard, A.; Fierro, V. Tannin/furanic foams without blowing agents and formaldehyde. Ind. Crops Prods. 2013, 49, 17–22. [Google Scholar] [CrossRef]
- Abdullah, U.H.; Pizzi, A.; Rode, K.; Delmotte, L.; Zhou, X.; Mansouri, H.R. Mimosa tannin resins for impregnated paper overlays. Eur. J. Wood Prod. 2013, 71, 153–162. [Google Scholar] [CrossRef]
- Tondi, G.; Pizzi, A. Tannin based rigid foams: Characterisation and modification. Ind. Crops Prod. 2009, 29, 356–363. [Google Scholar] [CrossRef]
- Letelier, M.; Makutkevic, J.; Padubskaya, A.; Plyusch, A.; Kuzhir, P.; Ivanoc, M.; Banys, J.; Pizzi, A.; Fierro, V.; Celzard, A. Tannin-based carbon foams for electromagnetic applications. Electromagnetic Compatibility. IEEE Trans. J. 2015, 57, 989–995. [Google Scholar]
- Basso, M.C.; Pizzi, A.; Al-Marzouki, F.; Abdalla, S. Horticultural/hydroponics and floral foams from tannins. Ind. Crops Prod. 2016, 87, 177–181. [Google Scholar] [CrossRef]
- Rangel, G.; Chapuis, H.; Basso, M.; Pizzi, A.; Delgado-Sanchez, C.; Fierro, V.; Celzard, A.; Gerardin, C. Improving water repellency and friability of tannin-furanic foams by oil-grafted flavonoid tannins. Bio. Resour. 2016, 11, 7754–7768. [Google Scholar]
- Melloua, F.; Loutrari, H.; Stamatis, H.; Roussos, C.; Kolisis, N. Enzymatic esterification of flavonoids with unsaturated fatty acids: Effect of the novel esters on vascular endothelial growth factor release from K562 cells. Process Biochem. 2006, 41, 2029–2034. [Google Scholar] [CrossRef]
- Milivojević, A.D.; Ćorović, M.M.; Simović, M.B.; .Banjanac, M.; Blagojević, S.N.; Pjanović, R.V.; Bezbradica, D.I. Novel Approach for Flavonoid Esters Production: Statistically Optimized Enzymatic Synthesis Using Natural Oils and Application in Cosmetics. Ind. Eng. Chem. Res. 2019, 58, 3640–3649. [Google Scholar] [CrossRef]
- Sun, C.Q.; Johnson, K.D.; Wong, H.; Foo, L.Y. Tansformation of Flavonoid Conjugates with Fatty Acids and Evaluations of Their Functionalities. Front. Pharmacol. 2017, 8, 759. [Google Scholar] [CrossRef] [Green Version]
- Grigsby, W.J.; Bridson, J.H.; Lomas, C.; Elliot, J.-A. Esterification of Condensed Tannins and Their Impact on the Properties of Poly(Lactic Acid). Polymers 2013, 5, 344–360. [Google Scholar] [CrossRef]
- Gaugler, M.; Grigsby, W.J. Thermal degradation of condensed tannins from radiata pine bark. J. Wood Chem. Technol. 2009, 29, 305–321. [Google Scholar] [CrossRef]
- Luo, C.; Grigsby, W.; Edmonds, N.; Easteal, A.; Al-Hakkak, J. Synthesis, characterization, and thermal behaviors of tannin stearates prepared from quebracho and pine bark extracts. J. Appl. Polym. Sci. 2010, 117, 352–360. [Google Scholar] [CrossRef]
- Bridson, J.H.; Grigsby, W.J.; Main, L. Synthesis and characterization of flavonoid laurate esters by transesterification. J. Appl. Polym. Sci. 2013, 129, 181–186. [Google Scholar] [CrossRef]
- Obounou Akong, F.; Pasc, A.; Mutlu, M.; Cosgun, S.; Gerardin-Charbonnier, C. Hydrogels obtained from an original catanionic system for efficient formulation of boron wood-preservatives. Intern. Biodeter. Biodegrad. 2013, 77, 123–126. [Google Scholar] [CrossRef]
- Obounou Akong, F.; Pasc, A.; Emo, M.; Gerardin-Charbonnier, C. A supramolecular hydrogel based on an original pseudopeptidic catanionic surfactant. New J. Chem. 2013, 37, 559–562. [Google Scholar] [CrossRef]
- Ortrand, R.U.; Ruhland, R.H.; Hüde, H.S.; Lembruch, W.S. Thermally Stable Rigid Foams Based on Isocyanate and Having Low Brittleness and Low Thermal Conductivity. US patent 6 284 812 B1, 2001. [Google Scholar]
- Martha, R.; Dirna, F.C.; Hasanusi, C.A.; Rahayu, I.S.; Darmawan, W. Surface free energy of 10 tropical woods species and their acrylic paint wettability. J. Adhesion Sci. Technol. 2020, 34, 167–177. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids, part VII in Cohesive Attraction of Solids and Fluids; Royal Society: London, UK, 1832. [Google Scholar]
- Dupré, A.M.; Dupré, P. Théorie Mécanique de la Chaleur; Gauthier-Villar: Paris, France, 1869. [Google Scholar]
- Delgado-Sanchez, C.; Letellier, M.; Fierro, V.; Chapuis, H.; Gerardin, C.; Pizzi, A.; Celzard, A. Hydrophobisation of tannin-based foams by covalent grafting of silanes. Ind. Crops Prod. 2016, 92, 116–126. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-polar surface tension of organic solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Prakash, C.K.; Mahadevan, K.M. Enhancing the properties of wood through chemical modification with palmitoyl chloride. Appl. Surface Sci. 2008, 254, 1751–1756. [Google Scholar] [CrossRef]
- Ping, L.; Pizzi, A.; Zhou, D.G.; Brosse, N. Condensed tannins from grape pomace: Characterization by FTIR and MALDI TOF and production of environment friendly wood adhesive. Ind. Crops Prod. 2012, 40, 13–20. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of Fourier Transform Infrared (FTIR) Spec-troscopy in the Characterization of Tannins. Appl. Spectroscopy Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Falcão, L.; Araújo, M.E.M. Tannins characterization in historic leathers by complementary analytical techniques ATR-FTIR, UV-Vis and chemical tests. J. Cultural Heritage 2013, 14, 499–508. [Google Scholar] [CrossRef]
- Selladurai, A.; Venujah, K. Modification of Tannin extracted from the bark of Acacia auriculiformis for the antibacte-rial activity and application of metal adsorption. Ruhuna J. Sci. 2017, 8, 90–102. [Google Scholar]
- Pasch, H.; Pizzi, A.; Rode, K. MALDI-TOF mass spectrometry of polyflavonoid tannins. Polymer 2001, 42, 7531–7539. [Google Scholar] [CrossRef]
- Drovou, S.; Pizzi, A.; Lacoste, C.; Zhang, J.; Abdalla, S.; Al-Marzouki, F.M. Flavonoid tannins linked to long carbohydrate chains—MALDI ToF analysis of the tannin extract of the african locust bean. Ind. Crops Prod. 2015, 67, 25–32. [Google Scholar] [CrossRef]
- Konai, N.; Pizzi, A.; Raidandi, D.; Lagel, M.C.; L’Hostis, C.; Saido, C.; Hamido, A.; Abdalla, S.; Bahabri, F.; Ganash, A. Aningre tannin extract characterisation and performance as an adhesive resin. Ind. Crops Prod. 2015, 77, 225–231. [Google Scholar] [CrossRef]
- Konai, N.; Raidandi, D.; Pizzi, A.; Meva’a, L. Characterisation of Ficus sycomorus using ATR-FTMIR, MALDI-TOF MS and 13C NMR methods. Eur. J. Wood Prod. 2017, 75, 807–815. [Google Scholar] [CrossRef]
- Bikoro Bi Athomo, A.; Engozogho Anris, S.P.; Safou-Tchiama, R.; Santiago-Medina, F.J.; Cabaret, T.; Pizzi, A.; Charrier, B. Chem-ical composition of African mahogany (K. ivorensis A. Chev) extractive and tannin structures of the bark by MALDI-TOF. Ind. Crops Prod. 2018, 113, 167–178. [Google Scholar] [CrossRef]












| Formulation | STD | A | B | C | D |
|---|---|---|---|---|---|
| Mimosa tannin extract (g) | 15 | 13.75 | 13.75 | 13.75 | 13.75 |
| Oil-grafted mimosa tannin (g) | - | 1.25 | 1.25 | 1.25 | 1.25 |
| Formaldehyde (g) | 3.7 | 3.7 | 0 | 0 | 0 |
| Furfuryl alcohol (g) | 6 | 5.5 | 5.5 | 5.5 | 6 |
| Water (g) | 2 | 0 | 0 | 0 | 2 |
| Glycerol (g) | 0 | 3 | 2.5 | 2.5 | 0 |
| pTSA (g) | 5.5 | 5.5 | 6 | 6 | 5.5 |
| Diethyl ether (g) | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 |
| Glutaraldehyde (g) | 0 | 0 | 3.7 | 0 | 0 |
| Acetaldehyde (g) | 0 | 0 | 0 | 3.7 | 3.7 |
| Fatty Acid Chloride | Yield of Oil-Grafted Tannin after Drying (g) |
|---|---|
| Lauryl | 7.60 |
| Palmitoyl | 7.85 |
| Oleyl | 8.36 |
| Mass Ratio T:P | Palmitoyl Chloride (g) | Chloroform (g) | Tannin (g) |
|---|---|---|---|
| 2:1 | 0.25 | 30 | 0.5 |
| 1:1 | 0.5 | 30 | 0.5 |
| 1:4 | 2 | 30 | 0.5 |
| 1:8 | 4 | 30 | 0.5 |
| Molecular Species | Experimental (Da) | Calculated (Da) | Remarks |
|---|---|---|---|
Fisetinidin-palmitate, protonated | 515 Da | 513 Da | Free flavonoid monoester |
Robinetinidin-palmitate, protonated, no Na+ | 529 Da | 528 Da | Free flavonoid monoester, protonated |
Gallocatechin-palmitate | 545 Da No Na+ | 544 Da No Na+ | Free flavonoid monoester, protonated |
Fisetinidin-(palmitate)2, no Na+ | 747 Da | 750 Da | Free flavonoid diester, deprotonated |
(Fisetinidin)2-palmitate  | 784 Da No Na+ 807 Da with Na+ | 784 Da No Na+ 807 Da with Na+ | Flavonoid dimer monoester |
| (Robinetidin)2-palmitate | 801 Da | 800 Da | Flavonoid dimer monoester, protonated |
Gallocatechin-(palmitate)2 + Na+ | 782 Da No Na+ 805 Da with Na+ | 782Da No Na+ 805 Da with Na+ | Free flavonoid diester |
(Fisetinidin)2-(palmitate)2 | 1021Da No Na+ 1046 Da with Na+ | 1021Da No Na+ 1046 Da with Na+ | Flavonoid Dimer diester |
| Robitenidin-(palmitate)3 | 1027 Da with Na+ | 1027 Da with Na+ | Flavonoid monomer triester |
| (Fisetinidin)2-(palmitate)3 | 1281 Da with Na+ | 1282 Da with Na+ | Flavonoid Dimer triester |
| Robinetinidin-fisetinidin-(palmitate)3 | 1297 Da with Na+ | 1298 Da with Na+ | Dimer triester |
| (Robinetinidin)2-(palmitate)3, protonated | 1315 Da with Na+ | 1314 Da with Na+ | Dimer triester |
| Robinetinidin-Gallocatechin-(palmitate)3, protonated | 1331 Da with Na+ | 1329 Da with Na+ | Dimer triester, |
| Glucose-(fisetinidin)2-(palmitate)3 | 1442 Da with Na+ | 1443 Da with Na+ | Glucoside dimer triester |
| Robinetinidin-(fisetinidin)2-(palmitate)3 | 1571 Da with Na+ | 1571 Da with Na+ | Trimer triester |
| (gallocatechin)2-(palmitate)4 | 1580 Da no Na+ | 1580Da no Na+ | Dimer tetraester |
| (Robinetinidin)2-fisetinidin-(palmitate)3 | 1587 Da with Na+ | 1587 Da with Na+ | Trimer triester |
| Foam Sample | Density (g/ |
|---|---|
| Standard foam | 0.12 |
| Formulation A | 0.0815 |
| Formulation B | 0.0853 |
| Formulation C | 0.0849 |
| Formulation D | 0.1061 |
| Sample | Contact Angle (°) | Surface Energies | ||
|---|---|---|---|---|
| Water | ||||
| STD | 91.83 (19.81) | 17.06 | 8.26 | 8.8 |
| A | 97.51 (3.89) | 13.76 | 7.91 | 5.85 |
| B | 99.93 (5.21) | 12.47 | 6.66 | 5.81 |
| C | 124.54 (7.07) | 3.41 | 3.94 | −0.53 |
| D | 116.44 (3.66) | 5.60 | 5.05 | 0.55 |
| Liquid | |||
|---|---|---|---|
| Water | 72.8 | 51 | 21.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azadeh, E.; Abdullah, U.H.; Ali, N.B.M.; Pizzi, A.; Gerardin-Charbonnier, C.; Gerardin, P.; Samiun, W.S.; Ashari, S.E. Development of Water Repellent, Non-Friable Tannin-Furanic-Fatty Acids Biofoams. Polymers 2022, 14, 5025. https://doi.org/10.3390/polym14225025
Azadeh E, Abdullah UH, Ali NBM, Pizzi A, Gerardin-Charbonnier C, Gerardin P, Samiun WS, Ashari SE. Development of Water Repellent, Non-Friable Tannin-Furanic-Fatty Acids Biofoams. Polymers. 2022; 14(22):5025. https://doi.org/10.3390/polym14225025
Chicago/Turabian StyleAzadeh, Elham, Ummi Hani Abdullah, Nurul Basirah Md Ali, Antonio Pizzi, Christine Gerardin-Charbonnier, Philippe Gerardin, Wan Sarah Samiun, and Siti Efliza Ashari. 2022. "Development of Water Repellent, Non-Friable Tannin-Furanic-Fatty Acids Biofoams" Polymers 14, no. 22: 5025. https://doi.org/10.3390/polym14225025
APA StyleAzadeh, E., Abdullah, U. H., Ali, N. B. M., Pizzi, A., Gerardin-Charbonnier, C., Gerardin, P., Samiun, W. S., & Ashari, S. E. (2022). Development of Water Repellent, Non-Friable Tannin-Furanic-Fatty Acids Biofoams. Polymers, 14(22), 5025. https://doi.org/10.3390/polym14225025