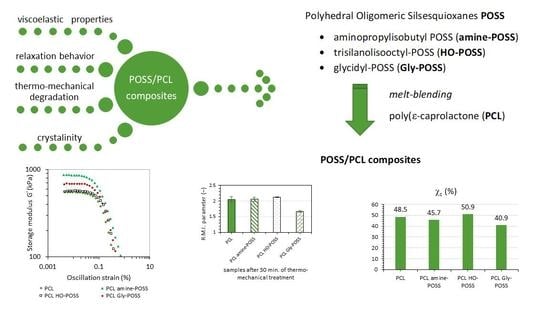
The Effect of Various Polyhedral Oligomeric Silsesquioxanes on Viscoelastic, Thermal Properties and Crystallization of Poly(ε-caprolactone) Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and PCL Mixture Preparation
2.2. Viscoelastic Properties at 25 °C and at Processing Temperature 100 °C
2.3. The DSC and TGA Analysis
3. Results and Discussion
3.1. The Dynamic Mechanical and Thermal Properties of PCL-POSS Composites at Ambient Temperature
3.2. The Viscoelastic Properties, Relaxation and Degradation of PCL-POSS at Temperature of 100 °C
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diaz Silvarrey, L.S.; Phan, A.N. Kinetic study of municipal plastic waste. Int. J. Hydrog. Energy 2016, 41, 16352–16364. [Google Scholar] [CrossRef] [Green Version]
- Steensgaard, I.M.; Syberg, K.; Rist, S.; Hartman, N.B.; Boldrin, A.; Hansen, S.F. From macro- to microplastic- Analysis of EU regulation along the life cycle of plastic bags. Environ. Pollut. 2017, 224, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filho, W.L.; Havea, P.H.; Balogun, A.-L.; Boenecke, J.; Maharaj, A.A.; Ha’apio, M.; Hemstock, S.L. Plastic debris on Pacific Islands: Ecological and health implications. Sci. Total Environ. 2019, 670, 181–187. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastics waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Barletta, M.; Aversa, C.; Puopolo, M.; Vesco, S. Extrusion blow molding of environmentally friendly bottles in biodegradable polyesters blends. Polym. Test. 2019, 77, 105885. [Google Scholar] [CrossRef]
- Przybysz-Romatowska, M.; Haponiuk, J.; Formela, K. Reactive extrusion of biodegradable aliphatic polyesters in the presence of free-radical-iniciators: A review. Polym. Degrad. Stab. 2020, 182, 109383. [Google Scholar] [CrossRef]
- Rydz, J.; Sikorska, W.; Kyulavska, M.; Christova, D. Polyester-based (bio)degradable polymers as environmentally friendly materials for sustainable development. Int. J. Mol. Sci. 2015, 16, 564–596. [Google Scholar] [CrossRef] [Green Version]
- Schindler, C.; Williams, B.I.; Patel, H.N.; Thomas, V. Electrospun polycaprolactone/polyglyconate blends: Miscibility, mechanical behavior, and degradation. Polymer 2013, 54, 6824–6833. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Zuhri, M.Y.M.; Norrahim, M.N.F.; Misenan, M.S.M.; Jenol, M.A.; Samsudin, S.A.; Nurazzi, N.M.; Asyraf, M.R.M.; Supian, A.B.M.; Bangar, S.P.; et al. Natural fiber-reinforced polycaprolactone green and hybrid biocomposites for various advanced applications. Polymers 2022, 14, 182. [Google Scholar] [CrossRef]
- Chawla, R.; Tan, A.; Maqsood, A.; Crowley, C.; Moiemen, N.S.; Cui, Z.; Butler, P.E.; Seifalian, A.M. A polyhedral oligomeric silsesquioxanes-based bilayered dermal scaffold seeded with adipose tissue-derived stem cells: In vitro assessment of biomechanical properties. J. Surg. Res. 2014, 188, 361–372. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, W.; Bartolo, P. Investigation of polycaprolactone for bone tissue engineering scaffolds: In vitro degradation and biological studies. Mater. Des. 2022, 216, 110582. [Google Scholar] [CrossRef]
- Bosworth, L.; Downes, S. Physicochemical characterization of degrading polycaprolactone scaffolds. Polym. Degrad. Stab. 2010, 95, 2269–2276. [Google Scholar] [CrossRef]
- Guerra, A.J.; Ciurana, J. Effect of fibre laser process on in-vitro degradation rate of a polycaprolactone stent a novel degradation study method. Polym. Degrad. Stab. 2017, 142, 42–49. [Google Scholar] [CrossRef]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.I. Electrospun polycaprolactone (PCL) degradation: An In Vitro and In Vivo study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef]
- Tous, I.; Ruseckaite, R.A.; Ciannamea, E.M. Suistanable hot-melt adhesives based on soybean protein isolate and polycaprolactone. Ind. Crop. Prod. 2019, 135, 153–158. [Google Scholar] [CrossRef]
- Laine, C.; Willberg-Keyriläinen, P.; Ropponen, J.; Liitiä, T. Lignin and lignin derivatives as components in biobased hot melt adhesives. J. Appl. Polym. Sci. 2019, 136, 47983. [Google Scholar] [CrossRef]
- Sarasam, A.R.; Krishnaswamy, R.K.; Madihally, S.V. Blending chitosan with polycaprolactone: Effects on physicochemical and antibacterial properties. Biomacromolecules 2006, 7, 1131–1138. [Google Scholar] [CrossRef]
- Nukala, S.G.; Kong, I.; Patel, V.I.; Kakarla, A.B.; Kong, W.; Buddrick, O. Development of biodegradable composites using polycaprolactone and bamboo powder. Polymers 2022, 14, 4169. [Google Scholar] [CrossRef] [PubMed]
- Richert, A.; Dąbrowska, G.B. Enzymatic degradation and biofilm formation during biodegradation of polylactide and polycaprolactone in various environments. Int. J. Bio. Macromol. 2021, 176, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.C.; Ibrahim, N.A.; Zainuddin, N.; Ariffin, H.; Yunus, W.M.Z.W.; Then, Y.Y. Enhancement of mechanical and dynamic mechanical properties of hydrophilic nanoclay reinforced polylactic acid/polycaprolactone/oil palm mesocarp fiber hybrid composites. Int. J. Polym. Sci. 2014, 2014, 715801. [Google Scholar] [CrossRef] [Green Version]
- Nezakati, T.; Tan, A.; Lim, J.; Cormia, R.D.; Teoh, S.-H.; Seifalian, A.M. Ultra-low percolation threshold POSS-PCL/graphene electrically conductive polymer. Neural tissue engineering nanocomposites for neurosurgery. Mater. Sci. Eng. C 2019, 104, 109915. [Google Scholar] [CrossRef]
- Sheng, L.; Jiang, R.; Zhu, Y.; Ji, Y. Electrospun cellulose nanocrystals/polycaprolactone composite fiber mats. J. Macromol. Sci. Part B Phys. 2014, 53, 820–828. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Huang, J.; Hao, M.; Hu, X.; Qian, X.; Fan, J.; Yang, H.; Yang, B. Influences of process parameters of near-field direct-writing melt electrospinning on performances of polycaprolactone/nano-hydroxyapatite scaffolds. Polymers 2022, 14, 3404. [Google Scholar] [CrossRef]
- Bikiaris, D.N. Nanocomposites of aliphatic polyesters: An overview of the effect of different nanofillers on enzymatic hydrolysis and biodegradation of polyesters. Polym. Degrad. Stab. 2013, 98, 1908–1928. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Pielichowski, K. Segmental dynamics in hybrid polymer/POSS nanomaterials. Prog. Polym. Sci. 2016, 52, 136–187. [Google Scholar] [CrossRef]
- Zhang, W.; Müller, A.H.E. Architecture, self-assembly and properties of well-defined hybrid polymers based on polyhedral oligomeric silsesquioxane (POSS). Prog. Polym. Sci. 2013, 38, 1121–1162. [Google Scholar] [CrossRef]
- Zhou, Z.; Cui, L.; Zhang, Y.; Zhang, Y.; Yin, N. Preparation and properties of POSS grafted polypropylene by reactive blending. Eur. Polym. J. 2008, 44, 3057–3066. [Google Scholar] [CrossRef]
- Alassod, A.; Islam, S.R.; Khalaji, M.S.; Tusiime, R.; Huang, W.; Xu, G. Polypropylene/lignin/POSS nanocomposites: Thermal and wettability properties, application in water remediation. Materials 2021, 14, 3950. [Google Scholar] [CrossRef]
- Rozga-Wijas, K.; Stanczyk, W.A.; Kurjata, J.; Kazimierski, S. Star-shaped and linear POSS-polylactide hybrid copolymers. Materials 2015, 8, 4400–4420. [Google Scholar] [CrossRef]
- Rybiński, P.; Syrek, B.; Bradło, D.; Żukowski, W. Effect of POSS particles and synergism action of POSS and poly-(melamine phosphate) on the thermal properties and flame retardance of silicone rubber composites. Materials 2018, 11, 1298. [Google Scholar] [CrossRef] [Green Version]
- Berthier, D.; Deffarges, M.-P.; Berton, N.; Venin, M.; Lacroix, F.; Schmaltz, B.; Tendron, Y.; Pestel, E.; Tran-Van, F.; Méo, S. POSS nanofiller-induced enhancement of the thermomechanical properties in a fluoroelastomer terpolymer. Materials 2018, 11, 1358. [Google Scholar] [CrossRef] [Green Version]
- Lipińska, M.; Imiela, M. Morphology, rheology and curing of (ethylene-propylene elastomer/hydrogenate acrylonitrile-butadiene rubber) blends reinforced by POSS and organoclay. Polym. Test. 2019, 75, 26–37. [Google Scholar] [CrossRef]
- Chruściel, J.J.; Leśniak, E. Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Xu, T.; Sima, H.; Hou, J. Effects of polyhedral oligomeric silsesquioxanes (POSS) on thermal and mechanical properties of polysiloxane foam. Materials 2020, 13, 4570. [Google Scholar] [CrossRef]
- Olejnik, A.; Sztorch, B.; Brząkalski, D.; Przekop, R.E. Silsesquioxanes in the cosmetic industry-applications and perspectives. Materials 2022, 15, 1126. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Gradzińska, K.; Marcinkowska, M.; Klajnert-Maculewicz, B.; Stanczyk, W.A. In Vitro studies of polyhedral oligo silsesquioxanes evidence for their low cytotoxicity. Materials 2015, 8, 6062–6070. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, H.; Cousins, B.G.; Seifalian, A.M. A nanocage for nanomedicine: Polyhedral oligomeric silsesquioxanes (POSS). Macromol. Rapid Commun. 2011, 32, 1032. [Google Scholar] [CrossRef]
- John, Ł. Selected developments and medical applications of organic-inorganic hybrid biomaterials based on functionalized spherosilicates. Mater. Sci. Eng. C 2018, 88, 172–181. [Google Scholar] [CrossRef]
- Raghunath, J.; Zhang, H.; Edirisinghe, M.J.; Darbyshire, A.; Butler, P.E.; Seifalian, A.M. A new biodegradable nanocomposite based on polyhedral oligomeric silsesquioxanes nanocage: Cytocompatibility and investigation into electrohydrodynamic jet fabrication techniques for tissue-engineered scaffolds. Biotechnol. Appl. Bioochem. 2009, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nezakati, T.; Tan, A.; Seifalian, A.M. Enhancing the electrical conductivity of a hybrid POSS-PCL/graphene nanocomposite polymer. J. Colloid Interface Sci 2014, 435, 145–155. [Google Scholar] [CrossRef]
- Han, D.; Wen, T.-J.; Han, G.; Deng, Y.-Y.; Deng, Y.; Zhang, Q.; Fu, Q. Synthesis of Janus POSS star polymer and exploring its compatibilization behavior for PLLA/PCL polymer blends. Polymer 2018, 136, 84–91. [Google Scholar] [CrossRef]
- Lee, K.S.; Chang, Y.W. Thermal and mechanical properties of poly(ε-caprolactone)/polyhedral oligomeric silsesquioxanes nanocomposites. Polym. Int. 2013, 62, 64–70. [Google Scholar] [CrossRef]
- Guan, W.; Qiu, Z. Isothermal crystallization kinetics, morphology, and dynamic mechanical properties of biodegradable poly(ε-caprolactone) and octavinyl-polyhedral oligomeric silsesquioxanes nanocomposites. Ind. Eng. Chem. Res. 2012, 51, 3203–3208. [Google Scholar] [CrossRef]
- Pan, H.; Jing, Y.; Qiu, Z. Crystallization and morphology studies of biodegradable poly(ε-caprolactone)/polyhedral oligomeric silsesquioxanes nanocomposites. Polym. Eng. Sci. 2011, 51, 2159–2165. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Zhang, W.; Zheng, S. Star-shaped poly(ε-caprolactone) with polyhedral oligomeric silsesquioxane core. Polymer 2006, 47, 6814–6825. [Google Scholar] [CrossRef]
- Lee, K.M.; Knight, P.T.; Chung, T.; Mather, P.T. Polycaprolactone-POSS chemical/physical double networks. Macromolecules 2008, 41, 4730–4738. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Martin-Alfonso, J.E. Thermal, thermo-oxidative and thermomechanical degradation of PLA: A comparative study based on rheological, chemical and thermal properties. Polym. Degrad. Stab. 2018, 150, 37–45. [Google Scholar] [CrossRef]
- Partal, P.; Martínez-Boza, F.; Conde, B.; Gallegos, C. Rheological characterisation of synthetic binders and unmodified bitumens. Fuel 1999, 78, 1–10. [Google Scholar] [CrossRef]
- Malkin, A.Y. Continuous relaxation spectrum—Its advantages and methods of calculation. Int. J. Appl. Mech. Eng. 2006, 11, 235–243. [Google Scholar]
- Bartenev, G.M.; Valishin, A.A.; Panchuk, I.I. Relaxation spectrometry of elastomers. Vysokomol. Soyed. 1977, A19, 187–193. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Vasilyev, G.B.; Adrianov, A.V. On continuous relaxation spectrum. Method of calculation. Polym. Sci. Ser. A 2010, 52, 1137–1141. [Google Scholar] [CrossRef]
- López-Barrón, C.R.; Macosko, C.W. Rheology of compatibilized immiscible blends with droplet-matrix and cocontinous morphologies during coarsening. J. Rheol. 2014, 58, 1935–1953. [Google Scholar] [CrossRef]
- Verney, V.; Michel, A. Influence de la polydispersité sur le comportement rhéologique á l’état fondu du polypropylene. Rheol. Acta 1985, 24, 627–631. [Google Scholar] [CrossRef]
- Marin, G.; Labaig, J.J.; Monge, P. Dynamic viscosity of entangled polymers. Polymer 1975, 16, 223–226. [Google Scholar] [CrossRef]
- Garcia-Franco, C.A.; Mead, D.W. Rheological and molecular characterization of linear backbone flexible polymers with the Cole-Cole model relaxation spectrum. Rheol. Acta 1999, 38, 34–47. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wang, L.; Ni, H.; Pittman, C.U., Jr. Polyhedral oligomeric silsesquioxanes (POSS) polymers and copolymers. A review. J. Inorg. Organomet. Polym. 2001, 11, 123–154. [Google Scholar] [CrossRef]
- Goudarzi, T.; Spring, D.W.; Paulino, G.H.; Lopez-Pamiez, O. Filled elastomers: A theory of filler reinforcement based on hydrodynamic and interphasial effect. J. Mech. Phys. Solids 2015, 80, 37–67. [Google Scholar] [CrossRef] [Green Version]
- Bautista, Y.; Gozalbo, A.; Mestre, S.; Sanz, V. Thermal degradation mechanism of a thermostable polyester stabilized with an open-cage oligomeric silsesquioxanes. Materials 2018, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Kodal, M.; Sirin, H.; Ozkoc, G. Effects of reactive and nonreactive POSS types on the mechanical, thermal, and morphological properties of plasticized poly(lactic acid). Polym. Eng. Sci. 2014, 54, 264–275. [Google Scholar] [CrossRef]











| Sample | Tg, °C | Tm, °C | Tc, °C | Hm, J g−1 | χc, % * |
|---|---|---|---|---|---|
| PCL | −70.93 | 56.90 | 32.63 | 65.10 | 48.5 |
| PCL amine-POSS | −63.00 | 57.43 | 34.24 | 61.34 | 45.7 |
| PCL HO-POSS | −63.33 | 56.37 | 31.55 | 68.39 | 50.9 |
| PCL Gly-POSS | −62.35 | 58.49 | 30.44 | 54.92 | 40.9 |
| Sample | T0.5%, °C | T1%, °C | T3%, °C | T5% °C | T50%, °C |
|---|---|---|---|---|---|
| PCL | 238 | 328 | 382 | 388 | 421 |
| PCL amine-POSS | 196 | 319 | 376 | 385 | 424 |
| PCL HO-POSS | 160 | 259 | 373 | 385 | 424 |
| PCL Gly-POSS | 187 | 304 | 376 | 385 | 424 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipińska, M. The Effect of Various Polyhedral Oligomeric Silsesquioxanes on Viscoelastic, Thermal Properties and Crystallization of Poly(ε-caprolactone) Nanocomposites. Polymers 2022, 14, 5078. https://doi.org/10.3390/polym14235078
Lipińska M. The Effect of Various Polyhedral Oligomeric Silsesquioxanes on Viscoelastic, Thermal Properties and Crystallization of Poly(ε-caprolactone) Nanocomposites. Polymers. 2022; 14(23):5078. https://doi.org/10.3390/polym14235078
Chicago/Turabian StyleLipińska, Magdalena. 2022. "The Effect of Various Polyhedral Oligomeric Silsesquioxanes on Viscoelastic, Thermal Properties and Crystallization of Poly(ε-caprolactone) Nanocomposites" Polymers 14, no. 23: 5078. https://doi.org/10.3390/polym14235078
APA StyleLipińska, M. (2022). The Effect of Various Polyhedral Oligomeric Silsesquioxanes on Viscoelastic, Thermal Properties and Crystallization of Poly(ε-caprolactone) Nanocomposites. Polymers, 14(23), 5078. https://doi.org/10.3390/polym14235078