Comparison of the Degradation Performance of Seven Different Choline Chloride-Based DES Systems on Alkaline Lignin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Elemental Analysis of Lignin
2.3. Characterization and Analysis of the Products
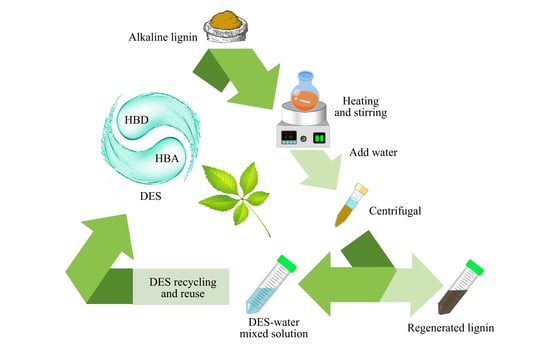
2.4. Lignin Depolymerization Process and DES Recycling
3. Results and Discussion
3.1. Determination of Phenolic Hydroxyl Groups
3.2. FT-IR Characterization
3.3. GPC Characterization
3.4. TG Characterization
3.5. 1H NMR Characterization
3.6. Recyclability of Solvents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Liu, J.; Zhao, L.; Liu, Z.; Liu, Q. Catalyst-free liquefaction of lignin for monophenols in hydrogen donor solvents. Fuel Process. Technol. 2022, 229, 107180. [Google Scholar] [CrossRef]
- Sheng, Y.; Ma, Z.; Wang, X.; Han, Y. Ethanol organosolv lignin from different agricultural residues: Toward basic structural units and antioxidant activity. Food Chem. 2022, 376, 131895. [Google Scholar] [CrossRef]
- Zhou, M.; Tang, C.; Li, J.; Xia, H.; Liu, P.; Xu, J.; Chen, C.; Jiang, J. Spherical NiCo-MOFs catalytic hydrogenolysis of lignin dimers and enzymatic lignin to value-added liquid fuels under nitrogen atmosphere. Fuel 2022, 315, 123156. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.C.; Hu, H.Q.; Xie, F.J.; Wei, X.Y.; Fan, X. Structural characterization of lignin and its degradation products with spectroscopic methods. J. Spectrosc. 2017, 2017, 8951658. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Lv, Z.W.; Rao, J.; Tian, R.; Sun, S.N.; Peng, F. Effects of hydrothermal pretreatment on the dissolution and structural evolution of hemicelluloses and lignin: A review. Carbohyd. Polym. 2022, 281, 119050. [Google Scholar] [CrossRef] [PubMed]
- Brebu, M.; Tamminen, T.; Spiridon, I. Thermal degradation of various lignins by TG-MS/FTIR and Py-GC-MS. J. Anal. Appl. Pyrol. 2013, 104, 531–539. [Google Scholar] [CrossRef]
- Prakash, D.G.; Gopinath, K.P.; Prasanth, S.M.; Harish, S.; Rishikesh, M.; Sivaramakrishnan, R.; Pugazhendhi, A. Extraction methodology of lignin from biomass waste influences the quality of bio-oil obtained by solvothermal depolymerization process. Chemosphere 2022, 293, 133473. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Q.; Pan, T.; Zuo, Y.; Fu, Y.; Guo, Q.X. Depolymerization of lignin by catalytic oxidation with aqueous polyoxometalates. Appl. Catal. A Gen. 2013, 467, 504–508. [Google Scholar] [CrossRef]
- Evstigneyev, E.I.; Shevchenko, S.M. Lignin valorization and cleavage of arylether bonds in chemical processing of wood: A mini-review. Wood Sci. Technol. 2020, 54, 787–820. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Qian, M.; Yadavalli, G. A thermal behavior and kinetics study of the catalytic pyrolysis of lignin. RSC Adv. 2016, 6, 100700–100707. [Google Scholar] [CrossRef]
- Lu, X.; Guo, H.; Chen, J.; Wang, D.; Lee, A.F.; Gu, X. Selective catalytic transfer hydrogenation of lignin to alkyl guaiacols over NiMo/Al-MCM-41. ChemSusChem 2022, 15, e202200099. [Google Scholar] [CrossRef]
- Shivhare, A.; Jampaiah, D.; Suresh, S.K.; Lee, A.F.; Srivastava, R.; Wilson, K. Hydrogenolysis of lignin-derived aromatic ethers over heterogeneous catalysts. ACS Sustain. Chem. Eng. 2021, 9, 3379–3407. [Google Scholar] [CrossRef]
- Sedai, B.; Díaz-Urrutia, C.; Baker, R.T.; Wu, R.; Silks, L.P.; Hanson, S.K. Aerobic oxidation of beta–1 lignin model compounds with copper and oxovanadium catalysts. ACS Catal. 2013, 3, 3111–3122. [Google Scholar] [CrossRef]
- Schutyser, W.; Kruger, J.S.; Robinson, A.M.; Katahira, R.; Brandner, D.G.; Cleveland, N.S.; Mittal, A.; Peterson, D.J.; Meilan, R.; Román-Leshkov, Y.; et al. Revisiting alkaline aerobic lignin oxidation. Green Chem. 2018, 20, 3828–3844. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Y.; Chen, X.; Wang, F.; Tian, X.; Gao, Y.; Zhang, Q. Deep eutectic solvent assists Bacillus australimaris to transform alkali lignin waste into small aromatic compounds. J. Clean. Prod. 2021, 320, 128719. [Google Scholar] [CrossRef]
- Sosa, F.H.B.; Abranches, D.O.; Da Costa Lopes, A.M.; Coutinho, J.A.P.; Da Costa, M.C. Kraft lignin solubility and its chemical modification in deep eutectic solvents. ACS Sustain. Chem. Eng. 2020, 8, 18577–18589. [Google Scholar] [CrossRef]
- Lou, R.; Ma, R.; Lin, K.; Ahamed, A.; Zhang, X. Facile extraction of wheat straw by deep eutectic solvent (DES) to produce lignin nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 10248–10256. [Google Scholar] [CrossRef]
- Li, L.; Wu, Z.; Liang, J.; Yu, L. Application of deep eutectic solvents in lignocellulosic biomass processing. J. For. Eng. 2020, 5, 20–28. [Google Scholar]
- Wang, S.; Li, Y.; Wen, X.; Fang, Z.; Zheng, X.; Di, J.; Li, H.; Li, C.; Fang, J. Experimental and theoretical study on the catalytic degradation of lignin by temperature-responsive deep eutectic solvents. Ind. Crops Prod. 2022, 177, 114430. [Google Scholar] [CrossRef]
- Hong, S.; Shen, X.J.; Pang, B.; Xue, Z.; Cao, X.F.; Wen, J.L.; Sun, Z.H.; Lam, S.S.; Yuan, T.Q.; Sun, R.C. In-depth interpretation of the structural changes of lignin and formation of diketones during acidic deep eutectic solvent pretreatment. Green Chem. 2020, 22, 1851–1858. [Google Scholar] [CrossRef]
- Hong, S.; Shen, X.J.; Sun, Z.H.; Yuan, T.Q. Insights into structural transformations of lignin toward high reactivity during choline chloride/formic acid deep eutectic solvents pretreatment. Front. Energy Res. 2020, 8, 573198. [Google Scholar] [CrossRef]
- Tan, Y.T.; Chua, A.S.M.; Ngoh, G.C. Evaluation on the properties of deep eutectic solvent-extracted lignin for potential aromatic bio-products conversion. Ind. Crops Prod. 2020, 154, 112729. [Google Scholar] [CrossRef]
- Li, L.; Wu, Z.; Xi, X.; Liu, B.; Hu, Y. A bifunctional bronsted acidic deep eutectic solvent to dissolve and catalyze the depolymerization of alkali lignin. J. Renew. Mater. 2021, 9, 219–235. [Google Scholar] [CrossRef]
- Hong, S.; Shen, X.J.; Xue, Z.; Sun, Z.; Yuan, T.Q. Structure-function relationships of deep eutectic solvents for lignin extraction and chemical transformation. Green Chem. 2020, 22, 7219–7232. [Google Scholar] [CrossRef]
- Yu, Q.; Song, Z.; Chen, X.; Fan, J.; Clark, J.H.; Wang, Z.; Sun, Y.; Yuan, Z. A methanol-choline chloride based deep eutectic solvent enhances the catalytic oxidation of lignin into acetovanillone and acetic acid. Green Chem. 2020, 22, 6415–6423. [Google Scholar] [CrossRef]
- Li, P.H.; Jiang, Z.W.; Li, J.Q.; Ren, J.P.; Wu, W.W. Research progress in quantitative determination of phenolic hydroxylgroups in lignin. Spectrosc. Spect. Anal. 2022, 42, 2666–2671. [Google Scholar] [CrossRef]
- Yang, W.S.; Jiao, L.; Wang, X.; Wu, W.B.; Lian, H.L.; Dai, H.Q. Formaldehyde-free self-polymerization of lignin-derived monomers for synthesis of renewable phenolic resin. Int. J. Biol. Macromol. 2021, 166, 1312–1319. [Google Scholar] [CrossRef]
- Li, P.; Jiang, Z.; Yang, C.; Ren, J.; Jiang, B.; Wu, W. Degradation of alkaline lignin in the lactic acid-choline chloride system under mild conditions. J. Renew. Mater. 2023. [Google Scholar] [CrossRef]
- Serrano, L.; Esakkimuthu, E.S.; Marlin, N.; Brochier-Salon, M.C.; Mortha, G.; Bertaud, F. Fast, easy, and economical quantification of lignin phenolic hydroxyl groups: Comparison with classical techniques. Energy Fuels 2018, 32, 5969–5977. [Google Scholar] [CrossRef]
- Nitsos, C.; Stoklosa, R.; Karnaouri, A.; Vörös, D.; Lange, H.; Hodge, D.; Crestini, C.; Rova, U.; Christakopoulos, P. Isolation and characterization of organosolv and alkaline lignins from hardwood and softwood biomass. ACS Sustain. Chem. Eng. 2016, 4, 5181–5193. [Google Scholar] [CrossRef]
- Xian, X.; Wu, S.; Wei, W.; Zhang, F. Pretreatment of kraft lignin by deep eutectic solvent and its utilization in preparation of lignin-based phenolic formaldehyde adhesive. BioResources 2021, 16, 3103–3120. [Google Scholar] [CrossRef]
- Li, W.X.; Xiao, W.Z.; Yang, Y.Q.; Wang, Q.; Chen, X.H.; Xiao, L.P.; Sun, R.C. Insights into bamboo delignification with acidic deep eutectic solvents pretreatment for enhanced lignin fractionation and valorization. Ind. Crops Prod. 2021, 170, 113692. [Google Scholar] [CrossRef]
- Zhang, S.; Su, L.; Liu, L.; Fang, G. Degradation on hydrogenolysis of soda lignin using CuO/SO42−/ZrO2 as catalyst. Ind. Crops Prod. 2015, 77, 451–457. [Google Scholar] [CrossRef]
- Xue, L.; Yan, L.; Cui, Y.; Jiang, M.; Xu, X.; Zhang, S.; Gou, J.; Zhou, Z. Degradation of lignin in ionic liquid with HCl as catalyst. Environ. Prog. Sustain. 2016, 35, 809–814. [Google Scholar] [CrossRef]
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcottc, M.; Zhang, X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chem. 2016, 18, 5133–5141. [Google Scholar] [CrossRef]
- Domínguez, J.C.; Santos, T.M.; Rigual, V.; Oliet, M.; Alonso, M.V.; Rodriguez, F. Thermal stability, degradation kinetics, and molecular weight of organosolv lignins from Pinus radiata. Ind. Crops Prod. 2018, 111, 889–898. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, M.; Li, X.; Zhang, B.; Jiao, M.; Chen, B. Promising and efficient lignin degradation versatile strategy based on DFT calculations. iScience 2022, 25, 103755. [Google Scholar] [CrossRef]
- Shen, X.J.; Chen, T.Y.; Wang, H.M.; Mei, Q.Q.; Yue, F.X.; Sun, S.N.; Wen, J.L.; Yuan, T.Q.; Sun, R.C. Structural and morphological transformations of lignin macromolecules during bio-based deep eutectic solvent (DES) pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 2130–2137. [Google Scholar] [CrossRef]
- Poletto, M. Assessment of the thermal behavior of lignins from softwood and hardwood species. Maderas Cienc. Tecnol. 2017, 19, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Ci, Y.; Yu, F.; Zhou, C.; Mo, H.; Li, Z.; Ma, Y.; Zang, L. New ternary deep eutectic solvents for effective wheat straw deconstruction into its high-value utilization under near-neutral conditions. Green Chem. 2020, 22, 8713–8720. [Google Scholar] [CrossRef]
- Hong, S.; Li, H.Y.; Shen, X.J.; Sun, S.M.; Sun, Z.H.; Yuan, T.Q. Unveiling the migration and transformation mechanism of lignin in eucalyptus during deep eutectic solvent pretreatment. ChemSusChem 2022, 15, e202200553. [Google Scholar] [CrossRef]
- Li, X.Y.; Guo, T.S.; Li, M.F.; Peng, F. Comparison of structure, thermal stability, and pyrolysis products of lignin extracted with ChCl-formic acid/lactic acid systems. J. Mater. Res. Technol. 2021, 14, 841–850. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Lou, Y.; Liu, Y.; Yu, H. Effect of lignin condensation on cellulose enzymatic hydrolysis during deep eutectic solvent fractionation of lignocellulose. J. For. Eng. 2021, 6, 101–108. [Google Scholar]
- Liu, R.; Ding, T.; Deng, P.; Yan, X.; Xiong, F.; Chen, J.; Wu, Z. Structure and properties of deep eutectic solvent lignin degraded by H2O2. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Jiang, J.; Zhang, Y.; Bi, S.; Wang, H.M. Revealing structural and functional specificity of lignin from tobacco stalk during deep eutectic solvents deconstruction aiming to targeted valorization. Ind. Crops Prod. 2022, 180, 114696. [Google Scholar] [CrossRef]
- Fernandes, C.; Melro, E.; Magalhães, S.; Alves, L.; Craveiro, R.; Filipe, A.; Valente, A.J.M.; Martins, G.; Antunes, F.E.; Romano, A.; et al. New deep eutectic solvent assisted extraction of highly pure lignin from maritime pine sawdust (Pinus pinaster Ait.). Int. J. Biol. Macromol. 2021, 177, 294–305. [Google Scholar] [CrossRef]
- Wu, M.; Liao, K.; Liu, C.; Yu, G.; Rahmaninia, M.; Li, H.; Li, B. Integrated and sustainable preparation of functional nanocellulose via formic acid/choline chloride solvents pretreatment. Cellulose 2021, 28, 9689–9703. [Google Scholar] [CrossRef]
- Oh, Y.; Park, S.; Jung, D.; Oh, K.K.; Lee, S.H. Effect of hydrogen bond donor on the choline chloride-based deep eutectic solvent-mediated extraction of lignin from pine wood. Int. J. Biol. Macromol. 2020, 165, 187–197. [Google Scholar] [CrossRef]



| Elements | Content [wt %] |
|---|---|
| C | 64.55 |
| H | 5.40 |
| N | 0.11 |
| S | 1.70 |
| Feedstock and Regenerated Lignin | Mw | Mn | Mw/Mn |
|---|---|---|---|
| AL | 7349 | 2041 | 3.6 |
| TA/CC | 4330 | 2509 | 1.7 |
| OX/CC | 2979 | 1894 | 1.6 |
| AA/CC | 3412 | 1699 | 2.0 |
| MA/CC | 3112 | 1858 | 1.7 |
| GE/CC | 2895 | 1743 | 1.7 |
| GC/CC | 2734 | 1708 | 1.6 |
| UR/CC | 2478 | 1284 | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Lu, Y.; Li, X.; Ren, J.; Jiang, Z.; Jiang, B.; Wu, W. Comparison of the Degradation Performance of Seven Different Choline Chloride-Based DES Systems on Alkaline Lignin. Polymers 2022, 14, 5100. https://doi.org/10.3390/polym14235100
Li P, Lu Y, Li X, Ren J, Jiang Z, Jiang B, Wu W. Comparison of the Degradation Performance of Seven Different Choline Chloride-Based DES Systems on Alkaline Lignin. Polymers. 2022; 14(23):5100. https://doi.org/10.3390/polym14235100
Chicago/Turabian StyleLi, Penghui, Yuan Lu, Xiaoyu Li, Jianpeng Ren, Zhengwei Jiang, Bo Jiang, and Wenjuan Wu. 2022. "Comparison of the Degradation Performance of Seven Different Choline Chloride-Based DES Systems on Alkaline Lignin" Polymers 14, no. 23: 5100. https://doi.org/10.3390/polym14235100
APA StyleLi, P., Lu, Y., Li, X., Ren, J., Jiang, Z., Jiang, B., & Wu, W. (2022). Comparison of the Degradation Performance of Seven Different Choline Chloride-Based DES Systems on Alkaline Lignin. Polymers, 14(23), 5100. https://doi.org/10.3390/polym14235100