Advances in Amine-Surface Functionalization of Inorganic Adsorbents for Water Treatment and Antimicrobial Activities: A Review
Abstract
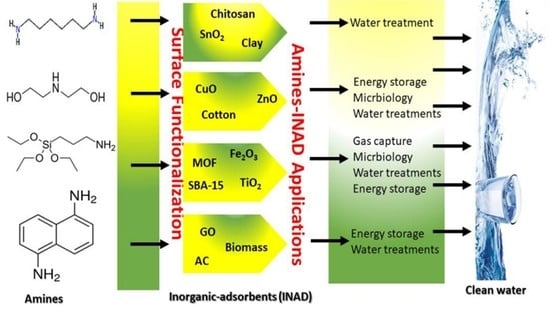
:1. Introduction
2. Amine for Surface Functionalization of Adsorbents
3. Effect of the Solvent
4. Effect of Temperature
5. Effect of the Concentration
6. Influence of Amines on the Hydrophilic Characters
7. Chemical State of the Amine Groups
8. Role of Amines in the Stability of Adsorbents
9. Pollutant Removal by Amine-Grafted Inorganic Adsorbents
9.1. Removal of Heavy Metals and Nitrates
9.2. Removal of Dyes
9.3. Elimination of Organic Pollutants
9.4. Degradation of Mixtures of Pollutants
10. Amines for Biomedical Applications
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| AEAPS | 3,2-aminoethylaminopropyltrimethoxysilane |
| APTES | 3-aminopropyltriethoxysilane |
| BDTA | benzyldimethyltetradecylammonium |
| CPTES | 3-chloropropyltriethoxysilane |
| DEA | Diethanolamine |
| DETA | Diethylenetriamine |
| EDA | Ethylenediamine |
| PEI | Polyethyleneimine |
| HMD | Hexamethylenediamine |
| DAN | 1,5-diaminonaphthalene |
| APTMS | 3-aminopropyltrimethoxysilane |
| DETA | Diethylenetriamine |
| PAMAM | Polyamidoamine-NH2 |
| MOFs | Metal–organic frameworks |
| ATP | 2-aminoterephthalic acid |
| 3APPA | 3-aminopropylphosphonic acid |
| INAD | Inorganic adsorbent |
| MOx | Metal oxide |
| TEPA | Tetraethylpentamine |
| NTA | Nitrilotriacetic acid |
| TETA | Triethylenetetraamine |
| PATP | p-aminothiophenol |
| PEHA | Pentaethylenehexamine |
| TEA | Trimethylamine |
| GO | Graphene oxide |
| AC | Activated carbon |
| 3APPA | 3-aminopropylphosphonic acid |
| 3PPA | 3-propylphosphonic acid |
References
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Ayad, M.M.; El-Nasr, A.A. Adsorption of cationic dye (methylene blue) from water using polyaniline nanotubes base. J. Phys. Chem. C 2010, 114, 14377–14383. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, X.; Li, Z.; Lei, L. Biodegradation of Reactive blue 13 in a two-stage anaerobic/aerobic fluidized beds system with a Pseudomonas sp. isolate. Bioresour. Technol. 2010, 101, 34–40. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Dutta, B.K. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. 2004, 112, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwar, K.; Osugi, M.; Chanmanee, W.; Chenthamarakshan, C.; Zanoni, M.V.B.; Kajitvichyanukul, P.; Krishnan-Ayer, R. Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 171–192. [Google Scholar] [CrossRef]
- Gözmen, B.; Kayan, B.; Gizir, A.M.; Hesenov, A. Oxidative degradations of reactive blue 4 dye by different advanced oxidation methods. J. Hazard. Mater. 2009, 168, 129–136. [Google Scholar] [CrossRef]
- Lazaratou, C.; Vayenas, D.; Papoulis, D. The role of clays, clay minerals and clay-based materials for nitrate removal from water systems: A review. Appl. Clay Sci. 2020, 185, 105377. [Google Scholar] [CrossRef]
- Lazaridis, N.; Asouhidou, D. Kinetics of sorptive removal of chromium(VI) from aqueous solutions by calcined Mg–Al–CO3 hydrotalcite. Water Res. 2003, 37, 2875–2882. [Google Scholar] [CrossRef]
- Suzaimi, N.D.; Goh, P.S.; Malek, N.A.N.N.; Lim, J.W.; Ismail, A.F. Performance of branched polyethyleneimine grafted porous rice husk silica in treating nitrate-rich wastewater via adsorption. J. Environ. Chem. Eng. 2019, 7, 103235. [Google Scholar] [CrossRef]
- Srivastava, V.; Choubey, A.K. Investigation of adsorption of organic dyes present in wastewater using chitosan beads immobilized with biofabricated CuO nanoparticles. J. Mol. Struct. 2021, 1242, 130749. [Google Scholar] [CrossRef]
- Abdulhameed, A.S.; Mohammad, A.-T.; Jawad, A.H. Application of response surface methodology for enhanced synthesis of chitosan tripolyphosphate/TiO2 nanocomposite and adsorption of reactive orange 16 dye. J. Clean. Prod. 2019, 232, 43–56. [Google Scholar] [CrossRef]
- Abdulhameed, A.S.; Jawad, A.H.; Mohammad, A.-T. Synthesis of chitosan-ethylene glycol diglycidyl ether/TiO2 nanoparticles for adsorption of reactive orange 16 dye using a response surface methodology approach. Bioresour. Technol. 2019, 293, 122071. [Google Scholar] [CrossRef] [PubMed]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic degradation for environmental applications—A review. J. Chem. Technol. Biotechnol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Tilt, B. The politics of industrial pollution in rural China. J. Peasant. Stud. 2013, 40, 1147–1164. [Google Scholar] [CrossRef]
- Antoniadou, M.; Panagiotopoulou, P.; Kondarides, D.I.; Lianos, P. Photocatalysis and photoelectrocatalysis using nanocrystalline titania alone or combined with Pt, RuO2 or NiO co-catalysts. J. Appl. Electrochem. 2012, 42, 737–743. [Google Scholar] [CrossRef]
- Nazari, P.; Gharibzadeh, S.; Ansari, F.; Nejand, B.A.; Eskandari, M.; Kohnehpoushi, S.; Ahmadi, V.; Salavati-Niasari, M. Facile green deposition of nanostructured porous NiO thin film by spray coating. Mater. Lett. 2017, 190, 40–44. [Google Scholar] [CrossRef]
- Bouazizi, N.; Bargougui, R.; Thebault, P.; Clamens, T.; Desriac, F.; Fioresi, F.; Ladam, G.; Morin-Grognet, S.; Mofaddel, N.; Lesouhaitier, O.; et al. Development of a novel functional core-shell-shell nanoparticles: From design to anti-bacterial applications. J. Colloid Interface Sci. 2018, 513, 726–735. [Google Scholar] [CrossRef]
- Nabil, B.; Morshed, M.N.; Ahmida, E.A.; Nemeshwaree, B.; Christine, C.; Julien, V.; Olivier, T.; Abdelkrim, A. Development of new multifunctional filter based nonwovens for organics pollutants reduction and detoxification: High catalytic and antibacterial activities. Chem. Eng. J. 2019, 356, 702–716. [Google Scholar] [CrossRef]
- Nabil, B.; Ahmida, E.A.; Christine, C.; Julien, V.; Abdelkrim, A. Polyfunctional cotton fabrics with catalytic activity and antibacterial capacity. Chem. Eng. J. 2018, 351, 328–339. [Google Scholar] [CrossRef]
- Bouazizi, N.; Vieillard, J.; Thebault, P.; Desriac, F.; Clamens, T.; Bargougui, R.; Couvrat, N.; Thoumire, O.; Brun, N.; Ladam, G.; et al. Silver nanoparticle embedded copper oxide as an efficient core–shell for the catalytic reduction of 4-nitrophenol and antibacterial activity improvement. Dalton Trans. 2018, 47, 9143–9155. [Google Scholar] [CrossRef] [PubMed]
- Vieillard, J.; Bouazizi, N.; Morshed, M.N.; Clamens, T.; Desriac, F.; Bargougui, R.; Thebault, P.; Lesouhaitier, O.; Le Derf, F.; Azzouz, A. CuO Nanosheets Modified with Amine and Thiol Grafting for High Catalytic and Antibacterial Activities. Ind. Eng. Chem. Res. 2019, 58, 10179–10189. [Google Scholar] [CrossRef]
- Fotsing, P.N.; Bouazizi, N.; Woumfo, E.D.; Mofaddel, N.; Le Derf, F.; Vieillard, J. Investigation of chromate and nitrate removal by adsorption at the surface of an amine-modified cocoa shell adsorbent. J. Environ. Chem. Eng. 2020, 9, 104618. [Google Scholar] [CrossRef]
- Bouazizi, N.; Vieillard, J.; Bargougui, R.; Couvrat, N.; Thoumire, O.; Morin, S.; Ladam, G.; Mofaddel, N.; Brun, N.; Azzouz, A.; et al. Entrapment and stabilization of iron nanoparticles within APTES modified graphene oxide sheets for catalytic activity improvement. J. Alloys Compd. 2019, 771, 1090–1102. [Google Scholar] [CrossRef]
- Bouazizi, N.; Boudharaa, T.; Bargougui, R.; Vieillard, J.; Ammar, S.; Le Derf, F.; Azzouz, A. Synthesis and properties of ZnO-HMD@ZnO-Fe/Cu core-shell as advanced material for hydrogen storage. J. Colloid Interface Sci. 2017, 491, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bouazizi, N.; Ajala, F.; Bettaibi, A.; Khelil, M.; Benghnia, A.; Bargougui, R.; Louhichi, S.; Labiadh, L.; Ben Slama, R.; Chaouachi, B.; et al. Metal-organo-zinc oxide materials: Investigation on the structural, optical and electrical properties. J. Alloys Compd. 2016, 656, 146–153. [Google Scholar] [CrossRef]
- Bouazizi, N.; Bargougui, R.; Boudharaa, T.; Khelil, M.; Benghnia, A.; Labiadh, L.; Ben Slama, R.; Chaouachi, B.; Ammar, S.; Azzouz, A. Synthesis and characterization of SnO 2-HMD-Fe materials with improved electric properties and affinity towards hydrogen. Ceram. Int. 2016, 42, 9413–9418. [Google Scholar] [CrossRef]
- Kumar, R.V.; Diamant, Y.; Gedanken, A. Sonochemical Synthesis and Characterization of Nanometer-Size Transition Metal Oxides from Metal Acetates. Chem. Mater. 2000, 12, 2301–2305. [Google Scholar] [CrossRef]
- An, W.-J.; Thimsen, E.; Biswas, P. Aerosol-Chemical Vapor Deposition Method For Synthesis of Nanostructured Metal Oxide Thin Films With Controlled Morphology. J. Phys. Chem. Lett. 2010, 1, 249–253. [Google Scholar] [CrossRef]
- Chequer, F.M.D.; de Oliveira, G.A.R.; Ferraz, E.R.A.; Cardoso, J.C.; Zanoni, M.V.B.; de Oliveira, D.P. Textile Dyes: Dyeing Process and Environmental Impact. Eco-Friendly Text. Dye. Finish. 2013, 6, 151–176. [Google Scholar]
- Cai, Z.; Sun, Y.; Liu, W.; Pan, F.; Sun, P.; Fu, J. An overview of nanomaterials applied for removing dyes from wastewater. Environ. Sci. Pollut. Res. 2017, 24, 15882–15904. [Google Scholar] [CrossRef]
- Mu, Y.; Rabaey, K.; Rozendal, R.A.; Yuan, Z.; Keller, J. Decolorization of Azo Dyes in Bioelectrochemical Systems. Environ. Sci. Technol. 2009, 43, 5137–5143. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Vieillard, J.; Bouazizi, N.; Bargougui, R.; Fotsing, P.N.; Thoumire, O.; Ladam, G.; Brun, N.; Hochepied, J.-F.; Woumfo, E.D.; Mofaddel, N.; et al. Metal-inorganic-organic core–shell material as efficient matrices for CO2 adsorption: Synthesis, properties and kinetic studies. J. Taiwan Inst. Chem. Eng. 2019, 95, 452–465. [Google Scholar] [CrossRef]
- Bargougui, R.; Bouazizi, N.; Brun, N.; Fotsing, P.N.; Thoumire, O.; Ladam, G.; Woumfo, E.D.; Mofaddel, N.; Le Derf, F.; Vieillard, J. Improvement in CO2 adsorption capacity of cocoa shell through functionalization with amino groups and immobilization of cobalt nanoparticles. J. Environ. Chem. Eng. 2018, 6, 325–331. [Google Scholar] [CrossRef]
- Vieillard, J.; Bouazizi, N.; Bargougui, R.; Brun, N.; Nkuigue, P.F.; Oliviero, E.; Thoumire, O.; Couvrat, N.; Woumfo, E.D.; Ladam, G.; et al. Cocoa shell-deriving hydrochar modified through aminosilane grafting and cobalt particle dispersion as potential carbon dioxide adsorbent. Chem. Eng. J. 2018, 342, 420–428. [Google Scholar] [CrossRef]
- Unnithan, M.R.; Vinod, V.P.; Anirudhan, T.S. Synthesis, Characterization, and Application as a Chromium(VI) Adsorbent of Amine-Modified Polyacrylamide-Grafted Coconut Coir Pith. Ind. Eng. Chem. Res. 2004, 43, 2247–2255. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Rijith, S.; Divya, L. Preparation and Application of a Novel Functionalized Coconut Coir Pith as a Recyclable Adsorbent for Phosphate Removal. Sep. Sci. Technol. 2009, 44, 2774–2796. [Google Scholar] [CrossRef]
- Tang, Y.; Liang, S.; Yu, S.; Gao, N.; Zhang, J.; Guo, H.; Wang, Y. Enhanced adsorption of humic acid on amine functionalized magnetic mesoporous composite microspheres. Colloids Surf. A Physicochem. Eng. Asp. 2012, 406, 61–67. [Google Scholar] [CrossRef]
- Jin, S.; Park, B.C.; Ham, W.S.; Pan, L.; Kim, Y.K. Effect of the magnetic core size of amino-functionalized Fe3O4-mesoporous SiO2 core-shell nanoparticles on the removal of heavy metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2017, 531, 133–140. [Google Scholar] [CrossRef]
- El-Dessouky, M.I.; Ibrahiem, H.H.; El-Masry, E.H.; El-Deen, G.E.S.; Sami, N.M.; Moustafa, M.E.; Mabrouk, E.M. Removal of Cs+ and Co2+ ions from aqueous solutions using poly (acrylamide-acrylic acid)/kaolin composite prepared by gamma radiation. Appl. Clay Sci. 2018, 151, 73–80. [Google Scholar] [CrossRef]
- Gardi, I.; Mishael, Y.G. Designing a regenerable stimuli-responsive grafted polymer-clay sorbent for filtration of water pollutants. Sci. Technol. Adv. Mater. 2018, 19, 588–598. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Morales, V.; Gardi, I.; Nir, S.; Undabeytia, T. Removal of pharmaceuticals from water by clay-cationic starch sorbents. J. Clean. Prod. 2018, 190, 703–711. [Google Scholar] [CrossRef]
- Kohay, H.; Izbitski, A.; Mishael, Y.G. Developing Polycation-Clay Sorbents for Efficient Filtration of Diclofenac: Effect of Dissolved Organic Matter and Comparison to Activated Carbon. Environ. Sci. Technol. 2015, 49, 9280–9288. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.-W.; Jeon, B.-H.; Chon, C.-M.; Kim, Y.; Schwartz, F.W.; Lee, E.-S.; Song, H. A novel chitosan/clay/magnetite composite for adsorption of Cu(II) and As(V). Chem. Eng. J. 2012, 200–202, 654–662. [Google Scholar] [CrossRef]
- Koushkbaghi, S.; Zakialamdari, A.; Pishnamazi, M.; Ramandi, H.F.; Aliabadi, M.; Irani, M. Aminated-Fe3O4 nanoparticles filled chitosan/PVA/PES dual layers nanofibrous membrane for the removal of Cr(VI) and Pb(II) ions from aqueous solutions in adsorption and membrane processes. Chem. Eng. J. 2018, 337, 169–182. [Google Scholar] [CrossRef]
- Guo, X.; Du, B.; Wei, Q.; Yang, J.; Hu, L.; Yan, L.-G.; Xu, W. Synthesis of amino functionalized magnetic graphenes composite material and its application to remove Cr(VI), Pb(II), Hg(II), Cd(II) and Ni(II) from contaminated water. J. Hazard. Mater. 2014, 278, 211–220. [Google Scholar] [CrossRef]
- Baghani, A.N.; Mahvi, A.H.; Gholami, M.; Rastkari, N.; Delikhoon, M. One-Pot synthesis, characterization and adsorption studies of amine-functionalized magnetite nanoparticles for removal of Cr (VI) and Ni (II) ions from aqueous solution: Kinetic, isotherm and thermodynamic studies. J. Environ. Health Sci. Eng. 2016, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Nonkumwong, J.; Ananta, S.; Srisombat, L. Effective removal of lead(ii) from wastewater by amine-functionalized magnesium ferrite nanoparticles. RSC Adv. 2016, 6, 47382–47393. [Google Scholar] [CrossRef]
- Zendehdel, M.; Ramezani, M.; Shoshtari-Yeganeh, B.; Cruciani, G.; Salmani, A. Simultaneous removal of Pb(II), Cd(II) and bacteria from aqueous solution using amino-functionalized Fe3O4/NaP zeolite nanocomposite. Environ. Technol. 2019, 40, 3689–3704. [Google Scholar] [CrossRef]
- Li, Z.; Pan, Z.; Wang, Y. Preparation of ternary amino-functionalized magnetic nano-sized illite-smectite clay for adsorption of Pb(II) ions in aqueous solution. Environ. Sci. Pollut. Res. 2020, 27, 11683–11696. [Google Scholar] [CrossRef] [PubMed]
- Morsi, R.E.; Al-Sabagh, A.M.; Moustafa, Y.M.; ElKholy, S.G.; Sayed, M.S. Polythiophene modified chitosan/magnetite nanocomposites for heavy metals and selective mercury removal. Egypt. J. Pet. 2018, 27, 1077–1085. [Google Scholar] [CrossRef]
- Hao, H.; Liu, G.; Wang, Y.; Shi, B.; Han, K.; Zhuang, Y.; Kong, Y. Simultaneous cationic Cu (II)–anionic Sb (III) removal by NH2-Fe3O4-NTA core-shell magnetic nanoparticle sorbents synthesized via a facile one-pot approach. J. Hazard. Mater. 2019, 362, 246–257. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Mira, H.; Abdel-Rahman, A.A.-H.; Guibal, E. Synthesis and adsorption characteristics of grafted hydrazinyl amine magnetite-chitosan for Ni(II) and Pb(II) recovery. Chem. Eng. J. 2019, 362, 310–324. [Google Scholar] [CrossRef] [Green Version]
- Feitoza, N.C.; Gonçalves, T.D.; Mesquita, J.J.; Menegucci, J.S.; Santos, M.-K.M.; Chaker, J.A.; Cunha, R.B.; Medeiros, A.; Rubim, J.C.; Sousa, M.H. Fabrication of glycine-functionalized maghemite nanoparticles for magnetic removal of copper from wastewater. J. Hazard. Mater. 2014, 264, 153–160. [Google Scholar] [CrossRef]
- Wei, W.; Desireddy, H.K.R.; Bediako, J.K.; Yun, Y.-S. Aliquat-336-impregnated alginate capsule as a green sorbent for selective recovery of gold from metal mixtures. Chem. Eng. J. 2016, 289, 413–422. [Google Scholar] [CrossRef]
- Bao, S.; Tang, L.; Li, K.; Ning, P.; Peng, J.; Guo, H.; Zhu, T.; Liu, Y. Highly selective removal of Zn(II) ion from hot-dip galvanizing pickling waste with amino-functionalized Fe3O4@SiO2 magnetic nano-adsorbent. J. Colloid Interface Sci. 2016, 462, 235–242. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.; Zhou, R.; Wang, C.; Jin, Y.; Qiu, J.; Hua, C.; Cao, Y. Synthesis of amino-functionalized bentonite/CoFe2O4@MnO2 magnetic recoverable nanoparticles for aqueous Cd2+ removal. Sci. Total Environ. 2019, 682, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A.; Adio, S.O.; Asif, M.; Dafalla, H. Statistical analysis of phenols adsorption on diethylenetriamine-modified activated carbon. J. Clean. Prod. 2018, 182, 960–968. [Google Scholar] [CrossRef]
- Peng, X.; Hu, F.; Zhang, T.; Qiu, F.; Dai, H. Amine-functionalized magnetic bamboo-based activated carbon adsorptive removal of ciprofloxacin and norfloxacin: A batch and fixed-bed column study. Bioresour. Technol. 2018, 249, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.N.; Bouazizi, N.; Behary, N.; Vieillard, J.; Thoumire, O.; Nierstrasz, V.; Azzouz, A. Iron-loaded amine/thiol functionalized polyester fibers with high catalytic activities: A comparative study. Dalton Trans. 2019, 48, 8384–8399. [Google Scholar] [CrossRef]
- Iannicelli-Zubiani, E.M.; Stampino, P.G.; Cristiani, C.; Dotelli, G. Enhanced lanthanum adsorption by amine modified activated carbon. Chem. Eng. J. 2018, 341, 75–82. [Google Scholar] [CrossRef]
- Mathew, A.; Parambadath, S.; Barnabas, M.J.; Song, H.J.; Kim, J.-S.; Park, S.S.; Ha, C.-S. Rhodamine 6G assisted adsorption of metanil yellow over succinamic acid functionalized MCM-41. Dye. Pigment. 2016, 131, 177–185. [Google Scholar] [CrossRef]
- Wu, P.; Dai, Y.; Long, H.; Zhu, N.; Li, P.; Wu, J.; Dang, Z. Characterization of organo-montmorillonites and comparison for Sr(II) removal: Equilibrium and kinetic studies. Chem. Eng. J. 2012, 191, 288–296. [Google Scholar] [CrossRef]
- Bauer, F.; Czihal, S.; Bertmer, M.; Decker, U.; Naumov, S.; Wassersleben, S.; Enke, D. Water-based functionalization of mesoporous siliceous materials, Part 1: Morphology and stability of grafted 3-aminopropyltriethoxysilane. Microporous Mesoporous Mater. 2017, 250, 221–231. [Google Scholar] [CrossRef]
- Xue, A.; Zhou, S.; Zhao, Y.; Lu, X.; Han, P. Effective NH2-grafting on attapulgite surfaces for adsorption of reactive dyes. J. Hazard. Mater. 2011, 194, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Monasterio, M.; Gaitero, J.J.; Erkizia, E.; Bustos, A.G.; Miccio, L.A.; Dolado, J.S.; Cerveny, S. Effect of addition of silica- and amine functionalized silica-nanoparticles on the microstructure of calcium silicate hydrate (C–S–H) gel. J. Colloid Interface Sci. 2015, 450, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; He, H.; Zhu, J.; Yuan, P.; Ma, Y.; Liang, X. Preparation and characterization of 3-aminopropyltriethoxysilane grafted montmorillonite and acid-activated montmorillonite. Sci. Bull. 2009, 54, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Demirbaş, Ö.; Alkan, M.; Doğan, M.; Turhan, Y.; Namli, H.; Turan, P. Electrokinetic and adsorption properties of sepiolite modified by 3-aminopropyltriethoxysilane. J. Hazard. Mater. 2007, 149, 650–656. [Google Scholar] [CrossRef]
- Ozmen, M.; Can, K.; Arslan, G.; Tor, A.; Cengeloglu, Y.; Ersoz, M. Adsorption of Cu(II) from aqueous solution by using modified Fe3O4 magnetic nanoparticles. Desalination 2010, 254, 162–169. [Google Scholar] [CrossRef]
- Tao, Q.; Fang, Y.; Li, T.; Zhang, D.; Chen, M.; Ji, S.; He, H.; Komarneni, S.; Zhang, H.; Dong, Y.; et al. Silylation of saponite with 3-aminopropyltriethoxysilane. Appl. Clay Sci. 2016, 132–133, 133–139. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, S.; Sun, W.; Li, Y. Selective adsorption of Pb(II) from aqueous solution using nanosilica functionalized with diethanolamine: Equilibrium, kinetic and thermodynamic. Microchem. J. 2019, 146, 270–278. [Google Scholar] [CrossRef]
- Xiong, Y.; Cui, X.; Wang, D.; Wang, Y.; Lou, Z.; Shan, W.; Fan, Y. Diethanolamine functionalized rice husk for highly efficient recovery of gallium(III) from solution and a mechanism study. Mater. Sci. Eng. C 2019, 99, 1115–1122. [Google Scholar] [CrossRef]
- Javadian, H.; Koutenaei, B.B.; Shekarian, E.; Sorkhrodi, F.Z.; Khatti, R.; Toosi, M. Application of functionalized nano HMS type mesoporous silica with N-(2-aminoethyl)-3-aminopropyl methyldimethoxysilane as a suitable adsorbent for removal of Pb (II) from aqueous media and industrial wastewater. J. Saudi Chem. Soc. 2017, 21, S219–S230. [Google Scholar] [CrossRef] [Green Version]
- Bouazizi, N.; Khelil, M.; Ajala, F.; Boudharaa, T.; Benghnia, A.; Lachheb, H.; Ben Slama, R.; Chaouachi, B.; M’Nif, A.; Azzouz, A. Molybdenum-loaded 1,5-diaminonaphthalene/ZnO materials with improved electrical properties and affinity towards hydrogen at ambient conditions. Int. J. Hydrogen Energy 2016, 41, 11232–11241. [Google Scholar] [CrossRef]
- Anyanwu, J.-T.; Wang, Y.; Yang, R.T. Influence of water on amine loading for ordered mesoporous silica. Chem. Eng. Sci. 2021, 241, 116717. [Google Scholar] [CrossRef]
- Gao, P.; Chen, D.; Chen, W.; Sun, J.; Wang, G.; Zhou, L. Facile synthesis of amine-crosslinked starch as an efficient biosorbent for adsorptive removal of anionic organic pollutants from water. Int. J. Biol. Macromol. 2021, 191, 1240–1248. [Google Scholar] [CrossRef]
- Zha, Q.; Sang, X.; Liu, D.; Wang, D.; Shi, G.; Ni, C. Modification of hydrophilic amine-functionalized metal-organic frameworks to hydrophobic for dye adsorption. J. Solid State Chem. 2019, 275, 23–29. [Google Scholar] [CrossRef]
- Ahmad, N.; Nordin, N.A.H.M.; Jaafar, J.; Malek, N.A.N.N.; Ismail, A.F.; Ramli, M.K.N. Modification of zeolitic imidazolate framework-8 with amine groups for improved antibacterial activity. Mater. Today Proc. 2021, 46, 2024–2029. [Google Scholar] [CrossRef]
- Xue, A.; Zhou, S.; Zhao, Y.; Lu, X.; Han, P. Adsorption of reactive dyes from aqueous solution by silylated palygorskite. Appl. Clay Sci. 2010, 48, 638–640. [Google Scholar] [CrossRef]
- Bertuoli, P.T.; Piazza, D.; Scienza, L.C.; Zattera, A.J. Preparation and characterization of montmorillonite modified with 3-aminopropyltriethoxysilane. Appl. Clay Sci. 2014, 87, 46–51. [Google Scholar] [CrossRef]
- Yang, S.-Q.; Yuan, P.; He, H.-P.; Qin, Z.-H.; Zhou, Q.; Zhu, J.; Liu, D. Effect of reaction temperature on grafting of γ-aminopropyl triethoxysilane (APTES) onto kaolinite. Appl. Clay Sci. 2012, 62–63, 8–14. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Li, Y.; Gao, E.; Cao, G.; Bernards, M.T.; He, Y.; Shi, Y. Enhanced SO2 Resistance of Tetraethylenepentammonium Nitrate Protic Ionic Liquid-Functionalized SBA-15 during CO2 Capture from Flue Gas. Energy Fuels 2020, 34, 8628–8634. [Google Scholar] [CrossRef]
- Choi, W.; Min, K.; Kim, C.; Ko, Y.S.; Jeon, J.W.; Seo, H.; Park, Y.-K.; Choi, M. Epoxide-functionalization of polyethyleneimine for synthesis of stable carbon dioxide adsorbent in temperature swing adsorption. Nat. Commun. 2016, 7, 12640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Choi, K.; Yu, H.J.; Won, Y.-J.; Kim, C.; Choi, M.; Cho, S.-H.; Lee, J.-H.; Lee, S.Y.; Lee, J.S. Thermal Stability Enhanced Tetraethylenepentamine/Silica Adsorbents for High Performance CO2 Capture. Ind. Eng. Chem. Res. 2018, 57, 4632–4639. [Google Scholar] [CrossRef]
- Takeuchi, M.; Martra, G.; Coluccia, S.; Anpo, M. Investigations of the Structure of H2O Clusters Adsorbed on TiO2 Surfaces by Near-Infrared Absorption Spectroscopy. J. Phys. Chem. B 2005, 109, 7387–7391. [Google Scholar] [CrossRef] [PubMed]
- Morterra, C. An infrared spectroscopic study of anatase properties. Part 6.—Surface hydration and strong Lewis acidity of pure and sulphate-doped preparations. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1988, 84, 1617–1637. [Google Scholar] [CrossRef]
- Paul, G.; Musso, G.E.; Bottinelli, E.; Cossi, M.; Marchese, L.; Berlier, G. Investigating the Interaction of Water Vapour with Aminopropyl Groups on the Surface of Mesoporous Silica Nanoparticles. ChemPhysChem 2017, 18, 839–849. [Google Scholar] [CrossRef]
- Iliade, P.; Miletto, I.; Coluccia, S.; Berlier, G. Functionalization of mesoporous MCM-41 with aminopropyl groups by co-condensation and grafting: A physico-chemical characterization. Res. Chem. Intermed. 2012, 38, 785–794. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Yang, J.; Yang, Q.; Li, C. Direct synthesis of highly ordered amine-functionalized mesoporous ethane-silicas. Microporous Mesoporous Mater. 2008, 109, 172–183. [Google Scholar] [CrossRef]
- Calvo, A.; Angelomé, P.; Sánchez, V.M.; Scherlis, D.A.; Williams, F.; Soler-Illia, G. Mesoporous Aminopropyl-Functionalized Hybrid Thin Films with Modulable Surface and Environment-Responsive Behavior. Chem. Mater. 2008, 20, 4661–4668. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Ishida, H.; Koenig, J.L. The structure of γ-aminopropyltriethoxysilane on glass surfaces. J. Colloid Interface Sci. 1980, 74, 396–404. [Google Scholar] [CrossRef]
- Gys, N.; Siemons, L.; Pawlak, B.; Wyns, K.; Baert, K.; Hauffman, T.; Adriaensens, P.; Blockhuys, F.; Michielsen, B.; Mullens, S.; et al. Experimental and computational insights into the aminopropylphosphonic acid modification of mesoporous TiO2 powder: The role of the amine functionality on the surface interaction and coordination. Appl. Surf. Sci. 2021, 566, 150625. [Google Scholar] [CrossRef]
- Wielant, J.; Hauffman, T.; Blajiev, O.; Hausbrand, R.; Terryn, H. Influence of the Iron Oxide Acid−Base Properties on the Chemisorption of Model Epoxy Compounds Studied by XPS. J. Phys. Chem. C 2007, 111, 13177–13184. [Google Scholar] [CrossRef]
- Gadois, C.; Światowska, J.; Zanna, S.; Marcus, P. Influence of Titanium Surface Treatment on Adsorption of Primary Amines. J. Phys. Chem. C 2013, 117, 1297–1307. [Google Scholar] [CrossRef]
- Abrahami, S.; Hauffman, T.; De Kok, J.M.; Mol, A.; Terryn, H. XPS Analysis of the Surface Chemistry and Interfacial Bonding of Barrier-Type Cr(VI)-Free Anodic Oxides. J. Phys. Chem. C 2015, 119, 19967–19975. [Google Scholar] [CrossRef]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering Hydrogel Adhesion for Biomedical Applications via Chemical Design of the Junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Prevoteau, A.; Elzière, P.; Hourdet, D.; Marcellan, A.; Leibler, L. Nanoparticle solutions as adhesives for gels and biological tissues. Nature 2014, 505, 382–385. [Google Scholar] [CrossRef]
- Azzouz, A.; Nousir, S.; Bouazizi, N.; Roy, R. Metal-Inorganic-Organic Matrices as Efficient Sorbents for Hydrogen Storage. ChemSusChem 2015, 8, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.U.H.; Khan, A.; Shah, A.; Wan, P.; Chen, Y.; Khan, G.M.; Khan, A.U.; Tahir, K.; Muhammad, N.; Khan, H.U. Enhanced photocatalytic and electrocatalytic applications of green synthesized silver nanoparticles. J. Mol. Liq. 2016, 220, 248–257. [Google Scholar] [CrossRef]
- Nasrollahzadeha, M.; Baran, T.; Baran, N.Y.; Sajjadia, M.; Tahsili, M.R.; Shokouhimehre, M. Pd nanocatalyst stabilized on amine-modified zeolite: Antibacterial and catalytic activities for environmental pollution remediation in aqueous medium. Sep. Purif. Technol. 2020, 239, 116542. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Q.; Yang, Z.; Wang, W. Adsorption of 2,4-D on magnetic graphene and mechanism study. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 509, 367–375. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Wang, J. A critical review of various adsorbents for selective removal of nitrate from water: Structure, performance and mechanism. Chemosphere 2021, 291, 132728. [Google Scholar] [CrossRef] [PubMed]
- Bakhta, S.; Sadaoui, Z.; Lassi, U.; Romar, H.; Kupila, R.; Vieillard, J. Performances of metals modified activated carbons for fluoride removal from aqueous solutions. Chem. Phys. Lett. 2020, 754, 137705. [Google Scholar] [CrossRef]
- Fotsing, P.N.; Woumfo, E.D.; Mezghich, S.; Mignot, M.; Mofaddel, N.; Le Derf, F.; Vieillard, J. Surface modification of biomaterials based on cocoa shell with improved nitrate and Cr(vi) removal. RSC Adv. 2020, 10, 20009–20019. [Google Scholar] [CrossRef]
- Dindar, M.H.; Yaftian, M.R.; Rostamnia, S. Potential of functionalized SBA-15 mesoporous materials for decontamination of water solutions from Cr(VI), As(V) and Hg(II) ions. J. Environ. Chem. Eng. 2015, 3, 986–995. [Google Scholar] [CrossRef]
- Guerra, D.; Mello, I.; Resende, R.; Silva, R. Application as absorbents of natural and functionalized Brazilian bentonite in Pb2+ adsorption: Equilibrium, kinetic, pH, and thermodynamic effects. Water Resour. Ind. 2013, 4, 32–50. [Google Scholar] [CrossRef] [Green Version]
- Marjanovic, V.; Lazarevic, S.; Jankovic-Castvan, I.; Jokic, B.; Bjelajac, A.; Janackovic, D.; Petrovic, R. Functionalization of thermo-acid activated sepiolite by amine-silane and mercapto-silane for chromium(VI) adsorption from aqueous solutions. Chem. Ind. 2013, 67, 715–728. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Majidi, M.; Zamani, A.; Liu, B. A review on the use of chitosan and chitosan derivatives as the bio-adsorbents for the water treatment: Removal of nitrogen-containing pollutants. Carbohydr. Polym. 2021, 273, 118625. [Google Scholar] [CrossRef]
- Gao, Y.; Ru, Y.; Zhou, L.; Wang, X.; Wang, J. Preparation and characterization of chitosan-zeolite molecular sieve composite for ammonia and nitrate removal. Compos. Adv. Mater. 2018, 27, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Lou, Z.; Zhang, W.; Hu, X.; Zhang, H. Synthesis of a novel functional group-bridged magnetized bentonite adsorbent: Characterization, kinetics, isotherm, thermodynamics and regeneration. Chin. J. Chem. Eng. 2017, 25, 587–594. [Google Scholar] [CrossRef]
- Araghi, S.H.; Entezari, M.H.; Chamsaz, M. Modification of mesoporous silica magnetite nanoparticles by 3-aminopropyltriethoxysilane for the removal of Cr(VI) from aqueous solution. Microporous Mesoporous Mater. 2015, 218, 101–111. [Google Scholar] [CrossRef]
- Morshed, M.N.; Bouazizi, N.; Behary, N.; Guan, J.; Nierstrasz, V. Stabilization of zero valent iron (Fe0) on plasma/dendrimer functionalized polyester fabrics for Fenton-like removal of hazardous water pollutants. Chem. Eng. J. 2019, 374, 658–673. [Google Scholar] [CrossRef]
- Laaz, I.; Stébé, M.-J.; Benhamou, A.; Zoubir, D.; Blin, J.-L. Influence of porosity and surface modification on the adsorption of both cationic and anionic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 30–40. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Mittal, D.A.; Saleh, T.; Nayak, A.; Agarwal, S.; Sikarwar, S. Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater. Sci. Eng. C 2012, 32, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Gupta, V.K. Column with CNT/magnesium oxide composite for lead(II) removal from water. Environ. Sci. Pollut. Res. 2012, 19, 1224–1228. [Google Scholar] [CrossRef]
- Gupta, V.; Srivastava, S.; Mohan, D.; Sharma, S. Design parameters for fixed bed reactors of activated carbon developed from fertilizer waste for the removal of some heavy metal ions. Waste Manag. 1998, 17, 517–522. [Google Scholar] [CrossRef]
- Mittal, A.; Mittal, J.; Malviya, A.; Kaur, D.; Gupta, V. Decoloration treatment of a hazardous triarylmethane dye, Light Green SF (Yellowish) by waste material adsorbents. J. Colloid Interface Sci. 2010, 342, 518–527. [Google Scholar] [CrossRef]
- Song, H.; Rioux, R.M.; Hoefelmeyer, J.; Komor, R.; Niesz, K.; Grass, M.; Yang, A.P.; Somorjai, G.A. Hydrothermal Growth of Mesoporous SBA-15 Silica in the Presence of PVP-Stabilized Pt Nanoparticles: Synthesis, Characterization, and Catalytic Properties. J. Am. Chem. Soc. 2006, 128, 3027–3037. [Google Scholar] [CrossRef]
- Panigrahi, S.; Basu, S.; Praharaj, S.; Pande, S.; Jana, S.; Pal, A.; Ghosh, S.K.; Pal, T. Synthesis and Size-Selective Catalysis by Supported Gold Nanoparticles: Study on Heterogeneous and Homogeneous Catalytic Process. J. Phys. Chem. C 2007, 111, 4596–4605. [Google Scholar] [CrossRef]
- Lu, H.; Yu, L.; Liu, Q.; Du, J. Ultrafine silver nanoparticles with excellent antibacterial efficacy prepared by a handover of vesicle templating to micelle stabilization. Polym. Chem. 2013, 4, 3448–3452. [Google Scholar] [CrossRef]
- Aditya, T.; Pal, A.; Pal, T. Nitroarene reduction: A trusted model reaction to test nanoparticle catalysts. Chem. Commun. 2015, 51, 9410–9431. [Google Scholar] [CrossRef]
- Bae, S.; Gim, S.; Kim, H.; Hanna, K. Effect of NaBH 4 on properties of nanoscale zero-valent iron and its catalytic activity for reduction of p-nitrophenol. Appl. Catal. B Environ. 2016, 182, 541–549. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, H.; Yang, X.; Zang, L. Tailoring Electronic Properties of Graphene by π–π Stacking with Aromatic Molecules. J. Phys. Chem. Lett. 2011, 2, 2897–2905. [Google Scholar] [CrossRef]
- Viswanathan, B.; Tanaka, K.; Toyoshima, I. Cluster model and charge transfer in a strong metal-support interaction (SMSI) state. Langmuir 1986, 2, 113–116. [Google Scholar] [CrossRef]
- Alighardashi, A.; Esfahani, Z.K.; Afkhami, A.; Najafi, F.; Hassan, N. Comparison of the efficiency of graphene oxide, activated graphene oxide, dendrimer-graphene oxide and activated dendrimer-graphene oxide for nitrate removal from aqueous solutions. Desalination Water Treat. 2017, 100, 100–115. [Google Scholar] [CrossRef]
- Yazdi, F.; Anbia, M.; Salehi, S. Characterization of functionalized chitosan-clinoptilolite nanocomposites for nitrate removal from aqueous media. Int. J. Biol. Macromol. 2019, 130, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.C.; Kim, J.Y.; Hwang, M.J.; Moon, H. Recovery of nitrate from water streams using amine-grafted and magnetized SBA-15. Korean J. Chem. Eng. 2018, 35, 489–497. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Wang, J.; Xu, S.; Yu, L.; Philippe, C.; Wintgens, T. Nitrate removal from water by new polymeric adsorbent modified with amino and quaternary ammonium groups: Batch and column adsorption study. J. Taiwan Inst. Chem. Eng. 2016, 66, 191–199. [Google Scholar] [CrossRef]
- Kheshti, Z.; Hassanajili, S. Novel Multifunctional Mesoporous Microsphere with High Surface Area for Removal of Zinc Ion from Aqueous Solution: Preparation and Characterization. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1613–1626. [Google Scholar] [CrossRef]
- Aguado, S.; Quirós, J.; Canivet, J.; Farrusseng, D.; Boltes, K.; Rosal, R. Antimicrobial activity of cobalt imidazolate metal–organic frameworks. Chemosphere 2014, 113, 188–192. [Google Scholar] [CrossRef]
- Zardini, H.Z.; Amiri, A.; Shanbedi, M.; Maghrebi, M.; Baniadam, M. Enhanced antibacterial activity of amino acids-functionalized multi walled carbon nanotubes by a simple method. Colloids Surf. B Biointerfaces 2012, 92, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Hanim, S.A.M.; Malek, N.A.N.N.; Ibrahim, Z. Amine-functionalized, silver-exchanged zeolite NaY: Preparation, characterization and antibacterial activity. Appl. Surf. Sci. 2016, 360, 121–130. [Google Scholar] [CrossRef]
- Furchtgott, L.; Wingreen, N.S.; Huang, K.C. Mechanisms for maintaining cell shape in rod-shaped Gram-negative bacteria. Mol. Microbiol. 2011, 81, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Kwakye-Awuah, B.; Williams, C.; Kenward, M.; Radecka, I. Antimicrobial action and efficiency of silver-loaded zeolite X. J. Appl. Microbiol. 2008, 104, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]























| INAD | Amines | Potential Application | Reference |
|---|---|---|---|
| Coconut coir pith | Amine-modified polyacrylamide | Removal of Cr(VI) | [36] |
| Coconut coir pith | Amino-functionalized polyacrylamide | Removal of phosphate | [37] |
| Microspheres (Fe3O4@mesoporous SiO2 core–shell composite microspheres) | Polyethyleneimine | Adsoption of humic acid (HA) Removal of heavy metals | [38,39] |
| Kaolin composite | Acrylamide | Removal of cesium and cobalt | [40] |
| Montmorillonite | Quarternized poly vinylpyridinium-co-styrene | Removal of selenate, potassium arsenate, methyl blue, eosin-Y, atrazine, and sulfentrazone | [41] |
| Polymer clay | Starch with quaternary ammonium groups | Removal of pharmaceuticals | [42] |
| Montmorillonite | Quarternized poly vinylpyridinium-co-styrene | Removal of the anionic pharmaceutical diclofenac (DCF) | [43] |
| Clay (heulandite) | Chitosan | Removal of Cu(II) and As(V) | [44] |
| Chitosan/PVA/PES | Fe3O4–NH2 | Removal of Cr(VI) | [45] |
| Magnetic graphene composite | 1,2-ethylenediamine | Removal of Cr(VI), Pb(II), Hg(II), Cd(II), and Ni(II) | [46] |
| Fe3O4 | 1,6-hexanediamine | Removal of Cr(VI) and Ni(II) ions | [47] |
| Magnesium ferrite nanoparticles (MgFe2O4) | Mesoporous amine NH2 | Removal of Pb(II) | [48] |
| Fe3O4/NaP zeolite nanocomposite | 3-aminopropyltrimethoxysilane | Removal of Pb(II), Cd(II), and bacteria | [49] |
| Magnetic illite–smectite clay | 3-aminopropyltriethoxysilane | Adsorption of Pb(II) ions | [50] |
| Magnetite nanocomposites | Chitosan nanoparticles and polythiophene | Removal of Hg(II) | [51] |
| Core–shell magnetic nanoparticles | 3-aminopropyltriethoxysilane and nitrilotriacetic acid | Removal of Cu(II) and Sb(III) | [52] |
| Chitosan-coated magnetite | Hydrazinyl amine | Removal of Ni(II) and Pb(II) | [53] |
| Maghemite nanoparticles | Glycine | Removal of Cu(II) | [54] |
| Magnetic nanoparticles | Dioctylphthalate triethylenetetraamine | Removal of Zn ions | [55] |
| Magnetic nanoparticles Fe3O4@SiO2 | 3-aminopropyltriethoxysilane | Removal of Zn(II) ions | [56] |
| Bentonite/CoFe2O4@MnO2 magnetite | 3-aminopropyltriethoxysilane | Removal of Cd2+ | [57] |
| Activated carbon, derived from waste rubber tires | Diethylenetriamine | Removal of phenol | [58] |
| Magnetic bamboo-based activated carbon | Ethylenediamine | Removal of ciprofloxacin and norfloxacin | [59] |
| Cocoa shell | Aminosilane | Reversible CO2 capture | [35] |
| Graphene oxide | 3-aminopropyltriethoxysilane | Reduction in 4-nitrophenol | [23] |
| Copper oxide (CuO) nanosheets | P-aminothiophenol and diethanolamine | Potential uses in catalysis and biomedical applications | [21] |
| Polyester fabrics (PET) | 3-aminopropyl triethoxysilane | Degradation of 4-nitrophenol (4-NP) and methylene blue | [60] |
| Cocoa shell | 3-aminopropyltriethoxysilane | Removal of chromate and nitrate | |
| Cocoa shell | 3-aminopropyltriethoxysilane | Desorption of CO2 | [34] |
| Activated carbon | pentaethylenehexamine | Removal of lanthanum | [61] |
| Amine Precursors | Solvents | Temperature (°C) | Grafting Strategies | Reference |
|---|---|---|---|---|
| APTES | Water | Room temperature | Conventional synthesis | [66] |
| APTES | Toluene | 45; 120 | Reflux | [65,68] |
| APTES | N,N-Dimethylformamide | 175 | Microwave-assisted synthesis | [68] |
| APTES | Water–ethanol mixture (25:75 vol.) | 80 | Conventional heating, reflux | [69] |
| APTES | Cyclohexane | 60 | Reflux | [70] |
| DEA | Ethanol | 70 | Reflux | [71] |
| DEA | Sodium carbonate solution | 80 | Conventional heating | [72] |
| 2-AEAPS | Hexane | (not mentioned) | Reflux | [73] |
| PAMAM | Hydroalcohol | 70 | Conventional heating | [18] |
| HMD | Water–ethanol mixture (25:75 vol.) | 80 | Conventional heating | [26] |
| DAN | Hydroalcohol | 80 | Conventional heating | [74] |
| APTMS | Toluene/water | 85 | Reflux | [75] |
| DETA | Epoxy Chloropropane/dimethylformamide | 95 | Conventional heating | [76] |
| ATP | Dichloromethane | 0 | Conventional cooling | [77] |
| TEA | Trimethylamine | Room temperature | Conventional synthesis | [78] |
| Inorganic Adsorbents (INADs) | Grafted Amines | Removed Pollutants | Adsorption Capacity (mg g−1) | Reference |
|---|---|---|---|---|
| Graphene oxide | PAMAM | Nitrate | 1025.9 | [126] |
| Porous rice husk silica | PEI | Nitrate | 94.5 | [9] |
| Nanochitosan/ clinoptilolite | PEHA | Nitrate | 277.8 | [127] |
| Magnetized mesoporous silica (SBA-15) | APTES | Nitrate | 44.9 | [128] |
| Polystyrene microspheres | hexamethylenetetramine | Nitrate | 221.8 | [129] |
| Cocoa shell | APTES | Nitrate Cr(IV) | 16.9 24.8 | [104] |
| Magnetic nanoparticules Fe3O4 SiO2 | APTES | Zn (II) | 270.3 | [130] |
| Mesoporous silica SBA-15 | APTES aminopropyl and N-propylsalicylaldimine | Cr (V) As (V) Hg (II) | 64.2 16.3 7.0 | [105] |
| Bentonite | APTES and 3,2-aminoAEAPS | Pb (II) Pb (II) | 27.6 29.5 | [106] |
| Acid-activated sepiolites | AEAPS | Cr (VI) | 60 | [107] |
| Kaolin composite | Acrylamide | Cs (II) Co(II) | 19.9 10.5 | [40] |
| Clay (heulandite) | Chitosan | Cu(II) As(V) | 17.2 5.9 | [44] |
| Chitosan/PVA/PES | Fe3O4–NH2 | Cr(VI) Pb(II) | 509.7 525.8 | [45] |
| Magnetic graphene composite | EDA | Cr(VI) Pb(II) Hg(II) Cd(II) Ni(II) | 17.3 27.9 23.0 27.9 22.0 | [46] |
| Fe3O4 | HMD | Cr (VI)\Ni (II) | 232.5 222.1 | [47] |
| Magnesium ferrite nanoparticles (MgFe2O4) | Mesoporous amine NH2 | Pb (II) | 135.1 | [48] |
| Fe3O4/NaP zeolite nanocomposite | APTES | Pb(II) Cd(II) | 181.8 50.2 | [49] |
| Magnetic illite–smectite clay | APTES | Pb(II) | 227.8 | [50] |
| Core–shell magnetic nanoparticle | APTES and NTA | Cu (II) Sb (III) | 55.6 51.1 | [52] |
| Chitosan-coated magnetite | Hydrazinyl amine | Ni(II) Pb(II) | 3.9 2.6 | [53] |
| Maghemite nanoparticles | Glycine | Cu (II) | 625 | [54] |
| Magnetic bentonite/Co Fe2O4@MnO2 | APTES | Cd(II) | 115.8 | [57] |
| Clay (palygorskite) | APTES | Reactive red 3BS | 34.2 | [79] |
| Bentonite | APTES-Fe3O4 | Methylene blue | 91.8 | [110] |
| Modified fibrous nonwoven | PAMAM | Methylene blue, malachite green | 49.5 47.0 | [18] |
| Mesoporous material SBA-15 | APTES | Red congo, brilliant green | 25.5 56.3 | [113] |
| Activated carbon | DETA | Phenol | 18.1 | [58] |
| Magnetic bamboo-based activated carbon | EDA | Ciprofloxacin, norfloxacin | 245.6 293.2 | [59] |
| Polyester fabrics (PET) | APTES PAMAM | 4-nitrophenol (4-NP) | 293.3 269.9 | [60] |
| Montmorillonite clay | Poly(4-vinylpyridine) | Selenate, Eosin Y dye methyl blue | 176.1 8.5 156.9 | [41] |
| Montmorillonite | Quarternized poly vinylpyridinium-co-styrene | Diclofenac | 100.6 | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouazizi, N.; Vieillard, J.; Samir, B.; Le Derf, F. Advances in Amine-Surface Functionalization of Inorganic Adsorbents for Water Treatment and Antimicrobial Activities: A Review. Polymers 2022, 14, 378. https://doi.org/10.3390/polym14030378
Bouazizi N, Vieillard J, Samir B, Le Derf F. Advances in Amine-Surface Functionalization of Inorganic Adsorbents for Water Treatment and Antimicrobial Activities: A Review. Polymers. 2022; 14(3):378. https://doi.org/10.3390/polym14030378
Chicago/Turabian StyleBouazizi, Nabil, Julien Vieillard, Brahim Samir, and Franck Le Derf. 2022. "Advances in Amine-Surface Functionalization of Inorganic Adsorbents for Water Treatment and Antimicrobial Activities: A Review" Polymers 14, no. 3: 378. https://doi.org/10.3390/polym14030378
APA StyleBouazizi, N., Vieillard, J., Samir, B., & Le Derf, F. (2022). Advances in Amine-Surface Functionalization of Inorganic Adsorbents for Water Treatment and Antimicrobial Activities: A Review. Polymers, 14(3), 378. https://doi.org/10.3390/polym14030378