Preparation and Characterization of Polymer Membranes Impregnated with Carbon Nanotubes for Olive Mill Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of OMW
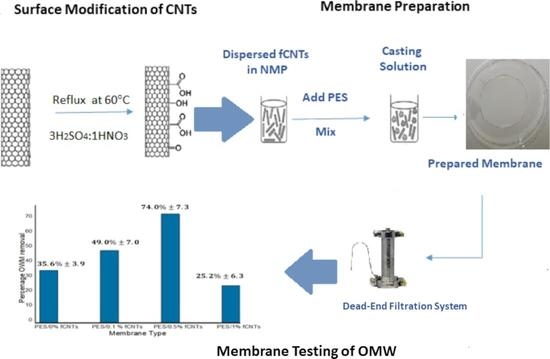
2.3. Surface Modification of CNTs
2.4. Membrane Preparation
2.5. Membrane Characterization
2.6. Membrane Performance
2.7. Determination of Total Phenolic Compounds
3. Results
3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2. X-ray Diffraction (XRD)
3.3. Scanning Electron Microscope (SEM)
3.4. Contact Angle Measurement
3.5. Porosity
3.6. Membrane Performance Testing
3.6.1. Pure Water Flux for Membranes
3.6.2. Rejection Percentages
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al Bawab, A.; Ghannam, N.; Abu-Mallouh, S.; Bozeya, A.; Abu-Zurayk, R.A.; Al-Ajlouni, Y.A.; Odeh, F.; Abu-Dalo, M.A. Olive mill wastewater treatment in Jordan: A Review. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 305, p. 012002. [Google Scholar]
- Coskun, T.; Debik, E.; Demir, N.M. Treatment of olive mill wastewaters by nanofiltration and reverse osmosis membranes. Desalination 2010, 259, 65–70. [Google Scholar] [CrossRef]
- Niaounakis, M.; Halvadakis, C.P. Olive Processing Waste Management: Literature Review and Patent Survey; Elsevier Science: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Ouzounidou, G.; Asfi, M. Determination of olive mill wastewater toxic effects on three mint species grown in hydroponic culture. J. Plant Nutr. 2012, 35, 726–738. [Google Scholar] [CrossRef]
- Khdair, A.I.; Abu-Rumman, G.; Khdair, S.I. Pollution estimation from olive mills wastewater in Jordan. Heliyon 2019, 5, e02386. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture. Annual Reports, Department of Agricultural Economy; Ministry of Agriculture: Amman, Jordan, 2014. [Google Scholar]
- Harwood, J. Handbook of Olive Oil: Analysis and Properties; Aparicio, R., Ed.; Gaithersburg, MD: Aspen, CO, USA, 2000; p. 620. [Google Scholar]
- Knop, A.; Böhmer, V.; Pilato, L.A. Phenol–Formaldehyde Polymers’. In Comprehensive Polymer Science and Supplements; Elsevier BV: Amsterdam, The Netherlands, 1989. [Google Scholar] [CrossRef]
- Abu-Dalo, M.; Abdelnabi, J.; Bawab, A.A. Preparation of Activated Carbon Derived from Jordanian Olive Cake and Functionalized with Cu/Cu2O/CuO for Adsorption of Phenolic Compounds from Olive Mill Wastewater. Materials 2021, 14, 6636. [Google Scholar] [CrossRef]
- Al-Bawab, A.; Ghannam, N.; Abu-Zurayk, R.A.; Odeh, F.; Bozeya, A.; Mallouh, S.A.; Al-Rawashdeh, N.A.; Abu-Dalo, M.A. Olive mill wastewater remediation by granular activated carbon impregnated with active materials. Fresenius Environ. Bull. 2018, 27, 2118–2126. [Google Scholar]
- Al-Bawab, A.; Alshawawreh, F.; Abu-Dalo, M.A.; Al-Rawashdeh, N.A.; Bozeya, A. Separation of soluble phenolic compounds from olive mill wastewater (OMW) using modified surfactant. Fresenius Environ. Bull. 2017, 26, 1949–1958. [Google Scholar]
- Choi, J.H.; Jegal, J.; Kim, W.N. Fabrication and characterization of multi-walled carbon nanotubes/polymer blend membranes. J. Membr. Sci. 2006, 284, 406–415. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Thanikaivelan, P.; Srinivasn, K.; Mohan, D.; Rajendran, M. Synthesis, characterization and thermal studies on cellulose acetate membranes with additive. Eur. Polym. J. 2004, 40, 2153–2159. [Google Scholar] [CrossRef]
- Daramola, M.; Sadare, O.; Oluwasina, O.; Iyuke, S. Synthesis and Application of Functionalized Carbon Nanotube Infused Polymer Membrane (fCNT/PSF/PVA) for Treatment of Phenol-Containing Wastewater. J. Membr. Sci. Res. 2019, 5, 310–316. [Google Scholar]
- Zhang, X.; Liu, Y.; Sun, C.; Ji, H.; Zhao, W.; Sun, S.; Zhao, C. Graphene oxide-based polymeric membranes for broad water pollutant removal. RSC Adv. 2015, 5, 100651–100662. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef]
- Barth, C.; Goncalves, M.C.; Pires, A.T.N.; Roeder, J.; Wolf, B.A. Asymmetric polysulfone and polyethersulfone membranes: Effects of thermodynamic conditions during formation on their performance. J. Membr. Sci. 2000, 169, 287–299. [Google Scholar] [CrossRef]
- Lau, W.W.; Guiver, M.D.; Matsuura, T. Phase separation in polysulfone/solvent/water and polyethersulfone/solvent/water systems. J. Membr. Sci. 1991, 59, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.L.; Zhao, J.Q.; Tang, W.; Pu, C.S. Hydrophilic modification of poly (ether sulfone) ultrafiltration membrane surface by self-assembly of TiO2 nanoparticles. Appl. Surf. Sci. 2005, 249, 76–84. [Google Scholar] [CrossRef]
- Son, M.; Choi, H.G.; Liu, L.; Celik, E.; Park, H.; Choi, H. Efficacy of carbon nanotube positioning in the polyethersulfone support layer on the performance of thin-film composite membrane for desalination. Chem. Eng. J. 2015, 266, 376–384. [Google Scholar] [CrossRef]
- Razmjou, A.; Resosudarmo, A.; Holmes, R.L.; Li, H.; Mansouri, J.; Chen, V. The effect of modified TiO2 nanoparticles on the polyethersulfone ultrafiltration hollow fiber membranes. Desalination 2012, 287, 271–280. [Google Scholar] [CrossRef]
- Badrinezhad, L.; Ghasemi, S.; Azizian-Kalandaragh, Y.; Nematollahzadeh, A. Preparation and characterization of polysulfone/graphene oxide nanocomposite membranes for the separation of methylene blue from water. Polym. Bull. 2018, 75, 469–484. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.; Xu, J.; Gao, C. Thin Film Composite Forward Osmosis Membrane with Single-Walled Carbon Nanotubes Interlayer for Alleviating Internal Concentration Polarization. Polymers 2020, 12, 260. [Google Scholar] [CrossRef] [Green Version]
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Ding, N.; Hu, X.; Shen, C.; Li, F.; Huang, M.; Wang, Z.; Sand, W.; Wang, C.C. Recent advances on electroactive CNT-based membranes for environmental applications: The perfect match of electrochemistry and membrane separation. Chem. Lett. 2020, 31, 2539–2548. [Google Scholar] [CrossRef]
- Peng, J.; He, Y.; Zhou, C.; Su, S.; Lai, B. The carbon nanotubes-based materials and their applications for organic pollutant removal: A critical review. Chin. Chem. Lett. 2021, 32, 1626–1636. [Google Scholar] [CrossRef]
- Celik, E.; Park, H.; Choi, H.; Choi, H. Carbon nanotube blended polyethersulfone membranes for fouling control in water treatment. Water Res. 2011, 45, 274–282. [Google Scholar] [CrossRef]
- Wang, L.; Song, X.; Wang, T.; Wang, S.; Wang, Z.; Gao, C. Fabrication and characterization of polyethersulfone/carbon nanotubes (PES/CNTs) based mixed matrix membranes (MMMs) for nanofiltration application. Appl. Surf. Science 2015, 330, 118–125. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mohammadi, T.; Bakhtiari, O. Effective treatment of dye wastewater via positively charged TETA-MWCNT/PES hybrid nanofiltration membranes. Sep. Purif. Technol. 2018, 194, 488–502. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Esteves, B.M.; Rodrigues, C.S.; Madeira, L.M. Synthetic olive mill wastewater treatment by Fenton’s process in batch and continuous reactors operation. Environ. Sci. Pollut. Res. 2018, 25, 34826–34838. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rinzler, A.G.; Dai, H.; Hafner, J.H.; Bradley, R.K.; Bould, P.J.; Lu, A.; Iverson, T.; Shelimov, K.; Huffman, C.B.; et al. Fullerene pipes. Science 1998, 280, 1253–1256. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, R.; Ge, Q.; Wang, H.; Xu, T. Preparation of polyethersulfone/carbon nanotube substrate for high-performance forward osmosis membrane. Desalination 2013, 330, 70–78. [Google Scholar] [CrossRef]
- John, B.I.J.U.; Sulaiman, C.T.; George, S.; Reddy, V.R.K. Total phenolics and flavonoids in selected medicinal plants from Kerala. Int. J. Pharm. Pharm. Sci. 2014, 6, 406–408. [Google Scholar]
- Nadour, M.; Boukraa, F.; Ouradi, A.; Benaboura, A. Effects of methylcellulose on the properties and morphology of polysulfone membranes prepared by phase inversion. Mater. Res. 2017, 20, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Kamil, A.M.; Hussein, F.H.; Halbus, A.F.; Bahnemann, D.W. Preparation, characterization, and photocatalytic applications of MWCNTs/TiO2 composite. Int. J. Photoenergy 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Abdulrazzak, F.H.; Alkiam, A.F.; Hussein, F.H. Behavior of X-ray analysis of carbon nanotubes. In Perspective of Carbon Nanotubes; IntechOpen: London, UK, 2019. [Google Scholar]
- Awasthi, K.; Kumar, R.; Raghubanshi, H.; Awasthi, S.; Pandey, R.; Singh, D.; Yadav, T.P.; Srivastava, O.N. Synthesis of nano-carbon (nanotubes, nanofibres, graphene) materials. Bull. Mater. Sci. 2011, 34, 607. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.B.; Lin, G.D.; Zhou, Z.H.; Dong, X.; Chen, T. Raman spectra of MWCNTs and MWCNT-based H2-adsorbing system. Carbon 2002, 40, 2429–2436. [Google Scholar] [CrossRef]
- Gohari, B.; Abu-Zahra, N. Polyethersulfone membranes prepared with 3-aminopropyltriethoxysilane modified alumina nanoparticles for Cu (II) removal from water. ACS Omega 2018, 3, 10154–10162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadek, E.M.; El-Nashar, D.E.; Ward, A.A.; Ahmed, S.M. Study on the properties of multi-walled carbon nanotubes reinforced poly (vinyl alcohol) composites. J. Polym. Res. 2018, 25, 1–13. [Google Scholar] [CrossRef]
- Qiu, S.; Wu, L.; Pan, X.; Zhang, L.; Chen, H.; Gao, C. Preparation and properties of functionalized carbon nanotube/PSF blend ultrafiltration membranes. J. Membr. Sci. 2009, 342, 165–172. [Google Scholar] [CrossRef]
- Blanco, J.F.; Sublet, J.; Nguyen, Q.T.; Schaetzel, P. Formation and morphology studies of different polysulfones-based membranes made by wet phase inversion process. J. Membr. Sci. 2006, 283, 27–37. [Google Scholar] [CrossRef]
- Bai, L.; Wu, H.; Ding, J.; Ding, A.; Zhang, X.; Ren, N.; Li, G.; Liang, H. Cellulose nanocrystal-blended polyethersulfone membranes for enhanced removal of natural organic matter and alleviation of membrane fouling. Chem. Eng. J. 2020, 382, 122919. [Google Scholar] [CrossRef]
- Sun, M.; Su, Y.; Mu, C.; Jiang, Z. Improved antifouling property of PES ultrafiltration membranes using additive of silica− PVP nanocomposite. Ind. Eng. Chem. Res. 2010, 49, 790–796. [Google Scholar] [CrossRef]
- Daramola, M.O.; Hlanyane, P.; Sadare, O.O.; Oluwasina, O.O.; Iyuke, S.E. Performance of carbon nanotube/polysulfone (CNT/Psf) composite membranes during Oil–water mixture separation: Effect of CNT dispersion method. Membranes 2017, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Rameetse, M.S.; Aberefa, O.; Daramola, M.O. Effect of loading and functionalization of carbon nanotube on the performance of blended polysulfone/polyethersulfone membrane during treatment of wastewater containing phenol and benzene. Membranes 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]













| Membrane Type | Porosity (%) |
|---|---|
| PES | 82.78 ± 0.03 |
| PES/0.1% fCNTs | 84.52 ± 0.02 |
| PES/0.5% fCNTs | 86.89 ± 0.01 |
| PES/1% fCNTs | 87.34 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Dalo, M.A.; Al-Atoom, M.A.; Aljarrah, M.T.; Albiss, B.A. Preparation and Characterization of Polymer Membranes Impregnated with Carbon Nanotubes for Olive Mill Wastewater. Polymers 2022, 14, 457. https://doi.org/10.3390/polym14030457
Abu-Dalo MA, Al-Atoom MA, Aljarrah MT, Albiss BA. Preparation and Characterization of Polymer Membranes Impregnated with Carbon Nanotubes for Olive Mill Wastewater. Polymers. 2022; 14(3):457. https://doi.org/10.3390/polym14030457
Chicago/Turabian StyleAbu-Dalo, Muna A., Maysa A. Al-Atoom, Mohannad T. Aljarrah, and Borhan A. Albiss. 2022. "Preparation and Characterization of Polymer Membranes Impregnated with Carbon Nanotubes for Olive Mill Wastewater" Polymers 14, no. 3: 457. https://doi.org/10.3390/polym14030457
APA StyleAbu-Dalo, M. A., Al-Atoom, M. A., Aljarrah, M. T., & Albiss, B. A. (2022). Preparation and Characterization of Polymer Membranes Impregnated with Carbon Nanotubes for Olive Mill Wastewater. Polymers, 14(3), 457. https://doi.org/10.3390/polym14030457