Preparation and Characterization of Diene Rubbers/Silica Composites via Reactions of Hydroxyl Groups and Blocked Polyisocyanates
Abstract
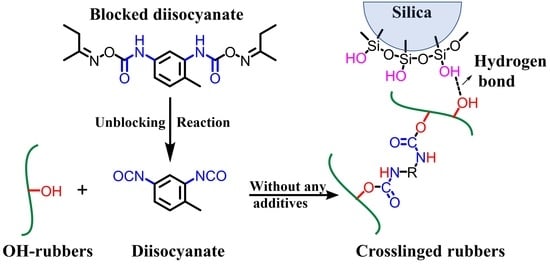
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of Hydroxylated BR and Hydroxylated SBR
2.1.2. Preparation of Blocked Polyisocyanates
2.1.3. Preparation of BROHx/BI/Silica and BR/S/Silica Composites
2.1.4. Preparation of SBROHx/BI/Silica and SBR/S/Silica Composites
2.2. Methods
3. Results
3.1. Hydroxyl Functionalization of Butadiene Rubber
3.2. Crosslinking of BROHx with Blocked Polyisocyanates
3.3. Mechanical Properties of BROHx/BI/Silica Composites
3.4. Mechanical Properties of SBROHx/BI/Silica Composites
3.5. Dispersion of Silica in the Rubber Matrix
3.6. Dynamic Mechanical Properties of SBROH5/Silica Composites Cured with Different Polyisocyanates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ikeda, Y.; Higashitani, N.; Hijikata, K.; Kokubo, Y.; Morita, Y.; Shibayama, M.; Osaka, N.; Suzuki, T.; Endo, H.; Kohjiya, S. Vulcanization: New focus on a traditional technology by small-angle neutron scattering. Macromolecules 2009, 42, 2741–2748. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Sulphur and peroxide vulcanisation of rubber compounds–overview. Chem. Pap. 2016, 70, 1533–1555. [Google Scholar] [CrossRef]
- Brostow, W.; Datashvili, T.; Hackenberg, K.P. Effect of different types of peroxides on properties of vulcanized EPDM+ PP blends. Polym. Compos. 2010, 31, 1678–1691. [Google Scholar] [CrossRef]
- Chokanandsombat, Y.; Sirisinha, C. MgO and ZnO as reinforcing fillers in cured polychloroprene rubber. J. Appl. Polym. Sci. 2013, 128, 2533–2540. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, P.; Dou, R.; Feng, S. Amine-functionalized POSS as cross-linkers of polysiloxane containing γ-chloropropyl groups for preparing heat-curable silicone rubber. Mater. Chem. Phys. 2018, 208, 19–27. [Google Scholar] [CrossRef]
- Tanrattanakul, V.; Kosonmetee, K.; Laokijcharoen, P. Polypropylene/natural rubber thermoplastic elastomer: Effect of phenolic resin as a vulcanizing agent on mechanical properties and morphology. J. Appl. Polym. Sci. 2009, 112, 3267–3275. [Google Scholar] [CrossRef]
- Heideman, G.; Noordermeer, J.W.; Datta, R.N.; van Baarle, B. Effect of zinc complexes as activator for sulfur vulcanization in various rubbers. Rubber Chem. Technol. 2005, 78, 245–257. [Google Scholar] [CrossRef]
- Heideman, G.; Noordermeer, J.W.; Datta, R.N.; van Baarle, B. Multifunctional additives as zinc-free curatives for sulfur vulcanization. Rubber Chem. Technol. 2006, 79, 561–588. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, Y.; Reinhard, M. Environmental and health impacts of artificial turf: A review. Environ. Sci. Technol. 2014, 48, 2114–2129. [Google Scholar] [CrossRef]
- Das, A.; Wang, D.-Y.; Leuteritz, A.; Subramaniam, K.; Greenwell, H.C.; Wagenknecht, U.; Heinrich, G. Preparation of zinc oxide free, transparent rubber nanocomposites using a layered double hydroxide filler. J. Mater. Chem. 2011, 21, 7194–7200. [Google Scholar] [CrossRef]
- Lin, T.; Zhang, X.; Tang, Z.; Guo, B. Renewable conjugated acids as curatives for high-performance rubber/silica composites. Green Chem. 2015, 17, 3301–3305. [Google Scholar] [CrossRef]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef]
- Basu, D.; Das, A.; Stöckelhuber, K.W.; Jehnichen, D.; Formanek, P.; Sarlin, E.; Vuorinen, J.; Heinrich, G. Evidence for an in situ developed polymer phase in ionic elastomers. Macromolecules 2014, 47, 3436–3450. [Google Scholar] [CrossRef]
- Pire, M.; Norvez, S.; Iliopoulos, I.; le Rossignol, B.; Leibler, L. Dicarboxylic acids may compete with standard vulcanisation processes for crosslinking epoxidised natural rubber. Compos. Interfaces 2014, 21, 45–50. [Google Scholar] [CrossRef]
- Pire, M.; Lorthioir, C.; Oikonomou, E.K.; Norvez, S.; Iliopoulos, I.; le Rossignol, B.; Leibler, L. Imidazole-accelerated crosslinking of epoxidized natural rubber by dicarboxylic acids: A mechanistic investigation using NMR spectroscopy. Polym. Chem. 2012, 3, 946–953. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Z.; Guo, B. Regulation of mechanical properties of diene rubber cured by oxa-Michael Reaction via manipulating network structure. Polymer 2018, 144, 57–64. [Google Scholar] [CrossRef]
- Wang, D.; Tang, Z.; Liu, Y.; Guo, B. Crosslinking diene rubbers by using an inverse vulcanised co-polymer. Green Chem. 2020, 22, 7337–7342. [Google Scholar] [CrossRef]
- Baker, C.; Barnard, D.; Porter, M. New reactions for the vulcanization of natural rubber. Rubber Chem. Technol. 1970, 43, 501–521. [Google Scholar] [CrossRef]
- Lautenschlaeger, F.; Myhre, M. Observations on the crosslinking of natural rubber with nitrosophenols and diisocyanates. Rubber Chem. Technol. 1974, 47, 100–117. [Google Scholar] [CrossRef]
- Kempermann, T. Sulfur-free vulcanization systems for diene rubber. Rubber Chem. Technol. 1988, 61, 422–447. [Google Scholar] [CrossRef]
- Parker, D.K.; Colvin, H.A.; Weinstein, A.H.; Chen, S.-L. Reactively curable rubbers—I: Diene elastomers with pendant isocyanate and/or hydroxyl functionality. Rubber Chem. Technol. 1990, 63, 582–598. [Google Scholar] [CrossRef]
- Ye, N.; Zheng, J.; Ye, X.; Xue, J.; Han, D.; Xu, H.; Wang, Z.; Zhang, L. Performance enhancement of rubber composites using VOC-Free interfacial silica coupling agent. Composites Part B 2020, 202, 108301. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, Z.; Guo, B.; Zhang, L. Concurrently improved dispersion and interfacial interaction in rubber/nanosilica composites via efficient hydrosilane functionalization. Compos. Sci. Technol. 2019, 169, 217–223. [Google Scholar] [CrossRef]
- Marković, G.; Marinović-Cincović, M.; Jovanović, V.; Samaržija-Jovanović, S.; Budinski-Simendić, J. NR/CSM/biogenic silica rubber blend composites. Compos. Part B 2013, 55, 368–373. [Google Scholar] [CrossRef]
- Ghoreishy, M.H.R.; Alimardani, M.; Mehrabian, R.Z.; Gangali, S.T. Modeling the hyperviscoelastic behavior of a tire tread compound reinforced by silica and carbon black. J. Appl. Polym. Sci. 2013, 128, 1725–1731. [Google Scholar] [CrossRef]
- Yatsuyanagi, F.; Suzuki, N.; Ito, M.; Kaidou, H. Effects of secondary structure of fillers on the mechanical properties of silica filled rubber systems. Polymer 2001, 42, 9523–9529. [Google Scholar] [CrossRef]
- Gui, Y.; Zheng, J.; Ye, X.; Han, D.; Xi, M.; Zhang, L. Preparation and performance of silica/SBR masterbatches with high silica loading by latex compounding method. Compos. Part B 2016, 85, 130–139. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Toyonaga, M.; Chammingkwan, P.; Terano, M.; Taniike, T. Well-defined polypropylene/polypropylene-grafted silica nanocomposites: Roles of number and molecular weight of grafted chains on mechanistic reinforcement. Polymers 2016, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Charness, M.E.; Simon, R.P.; Greenberg, D.A. Ethanol and the nervous system. N. Engl. J. Med. 1989, 321, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Lu, J.; Song, W.; Hu, J.; Han, B. Interfacial interaction modes construction of various functional SSBR–silica towards high filler dispersion and excellent composites performances. RSC Adv. 2019, 9, 18888–18897. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Wen, S.; Ma, H.; Li, Y.; Chen, L.; Wang, Z.; Yuan, B.; Liu, L. Improvement of silica dispersion in solution polymerized styrene–butadiene rubber via introducing amino functional groups. Ind. Eng. Chem. Res. 2018, 58, 1454–1461. [Google Scholar] [CrossRef]
- Weng, P.; Tang, Z.; Huang, J.; Wu, S.; Guo, B. Promoted dispersion of silica and interfacial strength in rubber/silica composites by grafting with oniums. J. Appl. Polym. 2019, 136, 48243. [Google Scholar] [CrossRef]
- Peng, C.-C.; Abetz, V. A simple pathway toward quantitative modification of polybutadiene: A new approach to thermoreversible cross-linking rubber comprising supramolecular hydrogen-bonding networks. Macromolecules 2005, 38, 5575–5580. [Google Scholar] [CrossRef]
- Yin, L.; Liu, Y.; Ke, Z.; Yin, J. Preparation of a blocked isocyanate compound and its grafting onto styrene-b-(ethylene-co-1-butene)-b-styrene triblock copolymer. Eur. Polym. 2009, 45, 191–198. [Google Scholar] [CrossRef]
- Payne, A.R. The dynamic properties of carbon black-loaded natural rubber vulcanizates. Part I. J. Appl. Polym. Sci. 1962, 6, 57–63. [Google Scholar] [CrossRef]












| Sample | Tensile Strength (MPa) | Modulus at 100% (MPa) | Modulus at 300% (MPa) | Elongation at Break (%) | Shore A Hardness | Permanent Set (%) | Resilience (%) | Compression Set (%) |
|---|---|---|---|---|---|---|---|---|
| BR/S | 10.4 | 1.0 | 1.9 | 1101 | 60 | 24 | 62.8 | 21.3 |
| BR/S/Si69 | 9.1 | 1.9 | 5.1 | 451 | 66 | 5 | 79.4 | 6.4 |
| BROH1/BI6 | 9.1 | 1.0 | 2.1 | 754 | 58 | 22 | 61.4 | 25.7 |
| BROH3/BI6 | 10.5 | 1.7 | 4.4 | 610 | 60 | 16 | 64.3 | 21.8 |
| BROH5/BI6 | 10.1 | 1.9 | 5.6 | 517 | 63 | 12 | 68.8 | 14.9 |
| Sample | Tensile Strength (MPa) | Modulus at 100% (MPa) | Modulus at 300% (MPa) | Elongation at Break (%) | Shore A Hardness | Permanent Set (%) | Resilience (%) | Compression Set (%) |
|---|---|---|---|---|---|---|---|---|
| SBR/S | 20.8 | 2.0 | 5.8 | 709 | 70 | 30 | 44.6 | 21.3 |
| SBR/S/Si69 | 21.6 | 4.0 | 15.3 | 401 | 76 | 9 | 51.4 | 11.1 |
| SBROH2/BI6 | 22.2 | 2.6 | 8.0 | 774 | 72 | 22 | 42.6 | 29.8 |
| SBROH5/BI6 | 23.2 | 3.0 | 13.2 | 505 | 76 | 15 | 44.2 | 23.1 |
| SBROH7/BI6 | 23.6 | 4.22 | 18.5 | 389 | 80 | 10 | 47.3 | 20.2 |
| Sample | Tensile Strength (MPa) | Modulus at 100% (MPa) | Modulus at 300% (MPa) | Elongation at Break (%) | Shore A Hardness | Permanent Set (%) | Resilience (%) | Compression Set (%) |
|---|---|---|---|---|---|---|---|---|
| SBROH5/B-HDI | 21.6 | 2.9 | 13.8 | 456 | 77 | 8 | 52.1 | 10.3 |
| SBROH5/B-PPDI | 21.0 | 3.2 | 15.0 | 391 | 76 | 8 | 52.9 | 8.9 |
| SBROH5/B-TDI | 21.9 | 3.5 | 18.2 | 351 | 77 | 5 | 53.2 | 8.3 |
| Sample | Tanδ at 0 °C | Tanδ at 60 °C |
|---|---|---|
| SBR/S | 0.16 | 0.13 |
| SBR/S/Si69 | 0.18 | 0.10 |
| SBROH2/BI6 | 0.17 | 0.17 |
| SBROH5/BI6 | 0.22 | 0.16 |
| SBROH7/BI6 | 0.41 | 0.16 |
| SBROH5/B-HDI | 0.19 | 0.13 |
| SBROH5/B-PPDI | 0.21 | 0.12 |
| SBROH5/B-TDI | 0.21 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, L.; Liu, Q. Preparation and Characterization of Diene Rubbers/Silica Composites via Reactions of Hydroxyl Groups and Blocked Polyisocyanates. Polymers 2022, 14, 461. https://doi.org/10.3390/polym14030461
Ge L, Liu Q. Preparation and Characterization of Diene Rubbers/Silica Composites via Reactions of Hydroxyl Groups and Blocked Polyisocyanates. Polymers. 2022; 14(3):461. https://doi.org/10.3390/polym14030461
Chicago/Turabian StyleGe, Lun, and Qiang Liu. 2022. "Preparation and Characterization of Diene Rubbers/Silica Composites via Reactions of Hydroxyl Groups and Blocked Polyisocyanates" Polymers 14, no. 3: 461. https://doi.org/10.3390/polym14030461
APA StyleGe, L., & Liu, Q. (2022). Preparation and Characterization of Diene Rubbers/Silica Composites via Reactions of Hydroxyl Groups and Blocked Polyisocyanates. Polymers, 14(3), 461. https://doi.org/10.3390/polym14030461