Synthesis, Characterization and Physicochemical Properties of Biogenic Silver Nanoparticle-Encapsulated Chitosan Bionanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
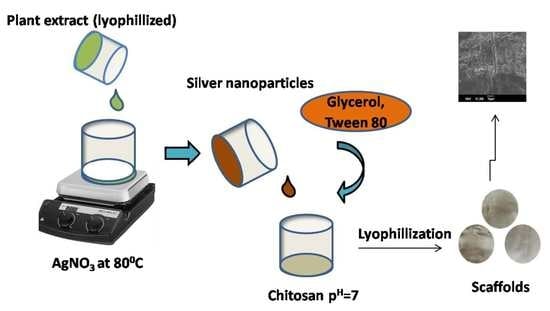
2.2.1. Plant Extract Preparation
2.2.2. Synthesis of Silver Nanoparticles (AgNPs)
2.2.3. Preparation of CH Scaffolds
2.3. Characterization
2.4. Evaluation of the Antioxidant Activity of AgNPs Using the DPPH Radical Scavenging Method
2.5. Antimicrobial Activity of AgNPs
2.6. Swelling Degree (%W)
- Ws—Initial weight of the scaffold sample (g).
- Wd—Final weight of the scaffold sample (g).
2.7. Water Vapor Transmission Rate (WVTR)
- Wi—Initial weight of scaffold sample (g).
- Wt—Final weight of scaffold sample (g).
- A—Mouth area of the bottle (m2).
- t—Time in h.
2.8. Test for Degradation in Hank’s Solution
- D2—Weight of the scaffolds after one week (g).
- D1—Dry weight of the scaffolds sample (g).
2.9. Test of Biodegradability
- Wf—Final weight of the scaffold (g).
- Wd—Dry weight of the scaffold (g).
2.10. Moisture Retention Capability (MRC)
- Wt—Weight of scaffold specimen at time t (g).
- Wi—Initial weight of the scaffold specimen (g).
3. Results and Discussions
3.1. Characterization of AgNPs
3.1.1. Ultraviolet (UV)–Visible Spectroscopy and Transmission Electron Microscopy (TEM) Analysis
3.1.2. X-ray Diffraction Studies
3.2. Antioxidant Activity of AgNPs
3.3. Antimicrobial Activity
3.4. Characterization of CH Scaffolds
3.5. Electrochemical Performance of the Fabricated CH-Ag NPS/GC (Chitosan-Silver Nanoparticles Coated on Glassy Carbon) in a Ferri/Ferro Probe
3.6. Evaluation of Mechanical Properties
3.7. Swelling Ratio of CH Scaffolds and Water Vapour Transmission Rate (WVTR)
3.8. Moisture Retention Capability (MRC) and Degradation in Hank’s Solution
3.9. Biodegradability of CH Scaffolds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Bionanocomposites: A new concept of ecological, bioinspired, and functional hybrid materials. Adv. Mater. 2007, 19, 1309–1319. [Google Scholar] [CrossRef]
- Pillai, S.K.; Ray, S.S. Chitosan-based nanocomposites. Nat. Polym. 2012, 2, 33–68. [Google Scholar]
- Ediyilyam, S.; George, B.; Shankar, S.S.; Dennise, T.T.; Wacławek, S.; Černík, M.; Padil, V.V.T. Chitosan/Gelatin/Silver Nanoparticles Composites Films for Biodegradable Food Packaging Applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Madihally, S.V.; Matthew, H.W.T. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- VandeVord, P.J.; Matthew, H.W.T.; DeSilva, S.P.; Mayton, L.; Wu, B.; Wooley, P.H. Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2002, 59, 585–590. [Google Scholar] [CrossRef]
- Rezaei, F.S.; Sharifianjazi, F.; Esmaeilkhanian, A.; Salehi, E. Chitosan films and scaffolds for regenerative medicine applications: A review. Carbohydr. Polym. 2021, 273, 118631. [Google Scholar] [CrossRef]
- Thein-Han, W.W.; Misra, R.D.K. Biomimetic chitosan–nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 1182–1197. [Google Scholar] [CrossRef]
- Kim, S.E.; Park, J.H.; Cho, Y.W.; Chung, H.; Jeong, S.Y.; Lee, E.B.; Kwon, I.C. Porous chitosan scaffold containing microspheres loaded with transforming growth factor-β1: Implications for cartilage tissue engineering. J. Control. Release 2003, 91, 365–374. [Google Scholar] [CrossRef]
- Mohandas, A.; Deepthi, S.; Biswas, R.; Jayakumar, R. Chitosan based metallic nanocomposite scaffolds as antimicrobial wound dressings. Bioact. Mater. 2018, 3, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural polymeric scaffolds in bone regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef]
- Kumari, S.; Singh, R.P.; Chavan, N.N.; Annamalai, P.K. Chitosan-based bionanocomposites for biomedical application. BioinspiredBiomim. Nanobiomater. 2016, 7, 219–227. [Google Scholar] [CrossRef]
- Soliman, H.; Elsayed, A.; Dyaa, A. Antimicrobial activity of silver nanoparticles biosynthesised by Rhodotorula sp. strain ATL72. Egypt. J. Basic Appl. Sci. 2018, 5, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P. Nano-TiO2 doped chitosan scaffold for the bone tissue engineering applications. Int. J. Biomater. 2018, 2018, 6576157. [Google Scholar] [CrossRef] [Green Version]
- Sudheesh Kumar, P.T.; Lakshmanan, V.-K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and microporous chitosan hydrogel/nanoZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef]
- Laohakunjit, N.; Noomhorm, A. Effect of plasticizers on mechanical and barrier properties of rice starch film. Starch-Stärke 2004, 56, 348–356. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.G. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.J.; Wang, D.Q. Dual effects of Tween 80 on protein stability. Int. J. Pharm. 2008, 347, 31–38. [Google Scholar] [CrossRef]
- Ziani, K.; Oses, J.; Coma, V.; Maté, J.I. Effect of the presence of glycerol and Tween 20 on the chemical and physical properties of films based on chitosan with different degree of deacetylation. LWT-Food Sci. Technol. 2008, 41, 2159–2165. [Google Scholar] [CrossRef]
- Shanthi, S.; Radha, R. Anti-microbial and Phytochemical Studies of Mussaenda frondosa Linn. Leaves. Pharmacogn. J. 2020, 12, 630–635. [Google Scholar] [CrossRef]
- Jayappa, M.D.; Ramaiah, C.K.; Kumar, M.A.P.; Suresh, D.; Prabhu, A.; Devasya, R.P.; Sheikh, S. Green synthesis of zinc oxide nanoparticles from the leaf, stem and in vitro grown callus of Mussaenda frondosa L.: Characterization and their applications. Appl. Nanosci. 2020, 10, 3057–3074. [Google Scholar] [CrossRef] [PubMed]
- Siju, E.N.; Rajalakshmi, G.R.; Kavitha, V.P.; Anju, J. In vitro antioxidant activity of Mussaenda frondosa. Int. J. Pharmtech Res. 2010, 2, 1236–1240. [Google Scholar]
- Bindhu, M.R.; Umadevi, M. Synthesis of monodispersed silver nanoparticles using Hibiscus cannabinus leaf extract and its antimicrobial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 101, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Xu, Q.; Li, X.; Chen, C.; Ma, L.; Li, S.; Che, Z.; Lin, H. Chitosan-based coating with antimicrobial agents: Preparation, property, mechanism, and application effectiveness on fruits and vegetables. Int. J. Polym. Sci. 2016, 2016, 4851730. [Google Scholar] [CrossRef] [Green Version]
- Damiri, F.; Bachra, Y.; Bounacir, C.; Laaraibi, A.; Berrada, M. Synthesis and characterization of lyophilized chitosan-based hydrogels cross-linked with benzaldehyde for controlled drug release. J. Chem. 2020, 2020, 8747639. [Google Scholar] [CrossRef]
- Cardenas, G.; Anaya, P.; Del Rio, R.; Schrebler, R.; Von Plessing, C.; Schneider, M. Scanning electron microscopy and atomic force microscopy of chitosan composite films. J. Chil. Chem. Soc. 2010, 55, 352–354. [Google Scholar] [CrossRef] [Green Version]
- Vicentini, D.S.; Smania, A.; Laranjeira, M.C.M. Chitosan/poly (vinyl alcohol) films containing ZnO nanoparticles and plasticizers. Mater. Sci. Eng. C 2010, 30, 503–508. [Google Scholar] [CrossRef]
- Sreelekha, E.; George, B.; Shyam, A.; Sajina, N.; Mathew, B. A Comparative Study on the Synthesis, Characterization, and Antioxidant Activity of Green and Chemically Synthesized Silver Nanoparticles. BioNanoScience 2021, 11, 489–496. [Google Scholar] [CrossRef]
- Shu, Y.; Xu, J.; Chen, J.; Xu, Q.; Xiao, X.; Jin, D.; Pang, H.; Hu, X. Ultrasensitive electrochemical detection of H2O2 in living cells based on ultrathin MnO2 nanosheets. Sens. Actuators B Chem. 2017, 252, 72–78. [Google Scholar] [CrossRef]
- Sivan, S.K.; Shankar, S.S.; KandambathPadinjareveetil, A.; Pilankatta, R.; Kumar, V.B.S.; Mathew, B.; George, B.; Makvandi, P.; Černík, M.; Padil, V.V.T. Fabrication of a Greener TiO2@Gum Arabic-Carbon Paste Electrode for the Electrochemical Detection of Pb2+ Ions in Plastic Toys. ACS Omega 2020, 5, 25390–25399. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef]
- Purwar, R.; Sharma, S.; Sahoo, P.; Srivastava, C.M. Flexible sericin/polyvinyl alcohol/clay blend films. Fibers Polym. 2015, 16, 761–768. [Google Scholar] [CrossRef]
- Rodríguez, M.; Osés, J.; Ziani, K.; Mate, J.I. Combined effect of plasticizers and surfactants on the physical properties of starch based edible films. Food Res. Int. 2006, 39, 840–846. [Google Scholar] [CrossRef]
- El-Hadi, A.M. Increase the elongation at break of poly (lactic acid) composites for use in food packaging films. Sci. Rep. 2017, 7, 46767. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.Y.; Wan, L.S.C. The effect of plasticizers on the properties of polyvinyl alcohol films. Drug Dev. Ind. Pharm. 1994, 20, 1007–1020. [Google Scholar] [CrossRef]
- Tao, L.; Zhonglong, L.; Ming, X.; Zezheng, Y.; Zhiyuan, L.; Xiaojun, Z.; Jinwu, W. In vitro and in vivo studies of a gelatin/carboxymethyl chitosan/LAPONITE® composite scaffold for bone tissue engineering. RSC Adv. 2017, 7, 54100–54110. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, S.K.; Chand, N.; Chaurasia, V. Investigation of water vapor permeability and antimicrobial property of zinc oxide nanoparticles-loaded chitosan-based edible film. J. Appl. Polym. Sci. 2010, 115, 674–683. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J.-L. Water and glycerol as plasticizers affect mechanical and water vapor barrier properties of an edible wheat gluten film. J. Food Sci. 1993, 58, 206–211. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, H.; Zhang, X.; Chen, Z.; Zhao, D.; Ma, J. A comparative study of two porous sponge scaffolds prepared by collagen derived from porcine skin and fish scales as burn wound dressings in a rabbit model. Regen. Biomater. 2020, 7, 63–70. [Google Scholar] [CrossRef]
- Epure, V.; Griffon, M.; Pollet, E.; Avérous, L. Structure and properties of glycerol-plasticized chitosan obtained by mechanical kneading. Carbohydr. Polym. 2011, 83, 947–952. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Effect of glycerol and corn oil on physicochemical properties of polysaccharide films–A comparative study. Food Hydrocoll. 2012, 27, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Cheng, J.; Yu, K. In situ reduction of silver nanoparticles by gelatin to obtain porous silver nanoparticle/chitosan composites with enhanced antimicrobial and wound-healing activity. Int. J. Biol. Macromol. 2019, 121, 633–642. [Google Scholar] [CrossRef]
- Kanimozhi, K.; Basha, S.K.; Kumari, V.S. Processing and characterization of chitosan/PVA and methylcellulose porous scaffolds for tissue engineering. Mater. Sci. Eng. C 2016, 61, 484–491. [Google Scholar] [CrossRef]
- Saral Sarojini, K.; Indumathi, M.P.; Rajarajeswari, G.R. Mahua oil-based polyurethane/chitosan/nanoZnO composite films for biodegradable food packaging applications. Int. J. Biol. Macromol. 2019, 124, 163–174. [Google Scholar] [CrossRef]
- Xiu, Z.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]








| Sample ID | Chitosan (CH) (% w/v) | Glycerol (% w/v) | Tween 80 (% w/v) | Silver Nanoparticles (AgNPs) (% w/v) |
|---|---|---|---|---|
| Cp | 2 | 0 | 0 | 0 |
| Cp1 | 2 | 0.05 | 0 | 0 |
| Cp2 | 2 | 0.05 | 0.05 | 0 |
| Cp3 | 2 | 0.05 | 0.05 | 0.001 |
| Cp4 | 2 | 0.05 | 0.05 | 0.0015 |
| Cp5 | 2 | 0.05 | 0.05 | 0.002 |
| Cp6 | 2 | 0.05 | 0.05 | 0.0025 |
| Sample | Tensile Strength (N/mm2) | % of EAB |
|---|---|---|
| Cp | 0.96 ± 0.02 | 4.63 ± 0.64 |
| Cp1 | 0.89 ± 0.04 | 17.94 ± 0.68 |
| Cp2 | 1.22 ± 0.04 | 6.39 ± 0.53 |
| Cp6 | 0.63 ± 0.05 | 10.51 ± 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ediyilyam, S.; Lalitha, M.M.; George, B.; Shankar, S.S.; Wacławek, S.; Černík, M.; Padil, V.V.T. Synthesis, Characterization and Physicochemical Properties of Biogenic Silver Nanoparticle-Encapsulated Chitosan Bionanocomposites. Polymers 2022, 14, 463. https://doi.org/10.3390/polym14030463
Ediyilyam S, Lalitha MM, George B, Shankar SS, Wacławek S, Černík M, Padil VVT. Synthesis, Characterization and Physicochemical Properties of Biogenic Silver Nanoparticle-Encapsulated Chitosan Bionanocomposites. Polymers. 2022; 14(3):463. https://doi.org/10.3390/polym14030463
Chicago/Turabian StyleEdiyilyam, Sreelekha, Mahesh M. Lalitha, Bini George, Sarojini Sharath Shankar, Stanisław Wacławek, Miroslav Černík, and Vinod Vellora Thekkae Padil. 2022. "Synthesis, Characterization and Physicochemical Properties of Biogenic Silver Nanoparticle-Encapsulated Chitosan Bionanocomposites" Polymers 14, no. 3: 463. https://doi.org/10.3390/polym14030463
APA StyleEdiyilyam, S., Lalitha, M. M., George, B., Shankar, S. S., Wacławek, S., Černík, M., & Padil, V. V. T. (2022). Synthesis, Characterization and Physicochemical Properties of Biogenic Silver Nanoparticle-Encapsulated Chitosan Bionanocomposites. Polymers, 14(3), 463. https://doi.org/10.3390/polym14030463