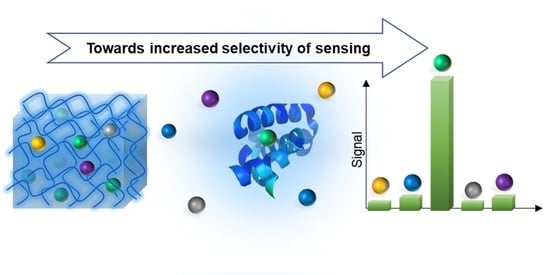
Shaping Macromolecules for Sensing Applications—From Polymer Hydrogels to Foldamers
Abstract
:1. Introduction
2. Hydrogel Materials in Sensing
2.1. Sensors Based on Natural Hydrogels
| Hydrogel | Sensing | Analyte | Characteristics | Ref. |
|---|---|---|---|---|
| Electrochemical Methods | ||||
| Polyacrylic acid-lignosulfonate-alginate-Ca2+ | Resistance | Strain | Resistance changes vs. time when monitoring different body joints motions, responsive performance up to 500 cycles. Compression stress—835 kPa Tensile fracture stress—357 kPa Stretching strain—1144% | [28] |
| BSA crosslinked by cysteine disulfide bridges | Amperometry | Physiological signals | Electrocardiography (ECG) for heart activity, electroencephalography (EEG) for brain activity, and electrooculography (EOG) for eye activity, conductivity = 5.3 mS cm−1 | [29] |
| Chitosan/cationic guar gum | Amperometry | Human body motions | 0.296 kPa pressure sensitivity when pressure was lower than 1.25 kP | [30] |
| Methacrylated-collagen, polypyrrole and glucose oxidase | Amperometry | Glucose | LOD = 2 mM, PBS buffer (pH = 7.4) LOD ≅ 200 mM in porcine meat Linear range: 0–4 mM High selectivity in vivo | [80] |
| Collagen from grass carp skin, graphene oxide and aptamer | Linear Sweep Voltammetry (LSV) | Dopamine | LOD = 0.75 nM, PBS buffer (pH = 7) Linear range: 1–1000 nM | [83] |
| Alginate copper oxide with glucose oxidase | Amperometry | Glucose | LOD = 1.6 µM in human serum Linear ranges: 0.04–3 mM and 4–35 mM Sensitivity: 30.443 and 7.025 µA mM−1 cm−2 Selectivity among ascorbic acid, uric acid, acetaminophen and phenylalanine | [93] |
| Chitosan crosslinked with silver ions | Linear Sweep Voltammetry (LSV) | Antioxidants (ascorbic acid) | Linear range: 0.04 µM–36 µM 0.1 mM H2O2 solution (pH = 4.5) Selectivity among glucose and sucrose | [82] |
| Chitosan crosslinked with genipin, amino-derived osmium redox complex and glucose oxidase | Amperometry | Glucose | Linear range: ~0.1–20 mM in PBS buffer (pH = 7) | [97] |
| Laponite-chitosan with lactate oxidase on glassy carbon electrode | Amperometry | L-lactate | LOD = 3.8 µM in alcoholic beverages Sensitivity: 0.326 A M−1 cm−2 LOQ = 12.6 µM Linear range: 10–70 µM | [98] |
| Chitosan, oxidized dextran, and CeO2/MnO2 hollow nanospheres | Amperometry | Glucose | LOD = 32.4 μM in PBS buffer (pH = 7.4) Sensitivity: 176 μA mM−1 cm−2 Linear range: 1–111 mM | [99] |
| 3-aminopropyltriethoxysilane/chitosan with glucose oxidase | Amperometry | Glucose | LOD = 0.2 µM, 0.10 M PBS buffer (pH = 7.0) Linear range: 0.2 µM–8.2mM and 0.2 µM–5.5 mM Sensitivity: 69.5 and 65 µA mM−1 cm−2 | [100] |
| Chitosan-carbon nanotubes (Chitosan-CNTs) | Cyclic Voltammetry (CV) | Dopamine | LOD = 2.00 vs. 1.00 µmol L−1 Sensitivity: 3.00 vs. 0.01 µA L µmol−1 (CNT loading 1.75% vs. 1%) Linear range: 0–10 µM for both in 300 μmol L−1 uric acid solution | [101] |
| Pectin/reduced graphene oxide | Cyclic Voltammetry (CV), Linear Sweep Voltammetry (LSV) | Dopamine, Paracetamol | LOD = 1.5 nM (Dopamine) LOQ = 0.4 nM (Dopamine) Linear range (LSV): 0.003–90.206 µM (Dopamine) LOD = 1.8 nM (Paracetamol) LOQ = 0.6 nM (Paracetamol) Linear range (LSV): 0.003–91.04 µM (Paracetamol) Both were performer in PBS (pH = 7.0) | [110] |
| Optical methods | ||||
| Pyrophosphate ion-alginate with carbon dots and Cu2+ | Fluorescence | Alkaline phosphatase (ALP) | LOD = 0.55 mU/mL Linear range: 0–100 mU/mL λem = 513 nm gel-sol transition | [61] |
| Collagen-lysyl oxidase | Fluorescence/Imaging | Biomarkers Lysyl oxidase | Turn-on fluorescence probe Extracellular matrix Before binding—ϕF = 0.09 λabs = 360 nm, λem = 395 nm After binding—ϕF = 0.89 λabs = 310 nm, λem = 455 nm | [76] |
| Human elastin-like polypeptide and bilirubin-binding protein UnaG | Fluorescence | Biomarkers, detection of bilirubin | Linear range: 0–100 nM in PBS buffer pH = 7.4 cell culture media; λem = 528 nm, λexc = 485 nm | [84] |
| Silk/Elastin-like recombinamers with fluorescent proteins (SELR-FPs) | Fluorescence | protein eqFP650 | λex = 475 nm, λem = 636 nm FRET pairs–fluorescent proteins AcEGFP and eqFP650, potential use as a biosensor | [85] |
| Gelatin methacryloyl | Tactile sensing | Pressure change | LOD = 0.1 Pa Sensitivity: 0.19 kPa−1 Durability up to 3000 cycles Suitable for wearable biosensing application | [86] |
| Gelatin-tannic acid | Volumetric | Mechanical change | Elongation 1600% Self-healing—0.65 s Self-healing efficiency—95% (Hydrogel combined with a resistor) | [87] |
| Gelatin crosslinked with carbon dots | Photoluminescence (PL) | pH | Increasing pH in range 3–10, Linear range: 5–7 pH PL quenching at λem = 431 nm, λex = 350 nm | [88] |
| Human serum albumin (HSA)-manganese complex | Magnetic resonance imaging (MRI) | pH | HSA-Mn2+ hydrogel capsule for in situ monitoring of gastric pH | [89] |
| Hyaluronic acid (HA) | Fluorescence | Hyaluronidase (HAse) | FRET-based quenching mechanism (FITC-donor, AuNPs–acceptor). Binding to HAse prevents FRET fluorescence quenching. LOD = 0.14 U/mL Linear range: 0.5–100 U/mL Selectivity among different ions (NaCl, KCl, MgSO4, CaCl2, small molecules (glutathione, glucose, glutamine and ascorbic acid), BSA, and enzymes (alkaline phosphatase, trypsin, papain). | [90] |
| Alginate crosslinked with Cu2+ | Fluorescence Immunoassay | Alkaline phosphatase (ALP) | LOD = 0.24 ng/mL (serum) Linear range: ~0–2 ng/mL Hepatis B surface antigen (HBsAg) Selectivity among Na+, K+, HAS, lysozyme, thrombin, glucose oxidase Gel-sol transition | [91] |
| Alginate-in-alginate with palladium tetracarboxyphenylporphyrin | Optical | Glucose | (low O2) Linear Range: 0.026–3.5 g/L Sensitivity: 97 ± 5.4 µs L/g (ambient O2) Linear Range: 0.87–3.5 g/L; Sensitivity: 7.5 ± 1.3 µs L/g | [92] |
| Alginate-based microfibres with mesoporous polyester beads | Optical | pH of epidermis | pH range: 4–9 (range for skin disorders and wounds variation) | [94] |
| Titanium oxide nanotubes/alginate hydrogel | Colorimetric assay | Biomarkers | LOD = 0.069 mM (lactate) Linear ranges: 0.1–1.0 mM LOD = 0.044 mM (glucose) Linear range: 0.1–0.8 mM | [95] |
| Fluorescent chitosan | Fluorescence | Nitrocompounds, p-nitrophenol | Nitrocompounds quench Fluorescence, LOD = 0.35–2.30 µM (2,4,6-trinitrophenol) LOD = 0.90–5.30 µM (p-nitrophenol) | [96] |
| Extract grape skin/tara gum, cellulose nanocrystal | Absorbance (Color change) | pH | Intensity decreases when pH increases pH range: 1–11 pH in range 1–5 λmax = 528 nm. pH in range 6–10 λmax = 618 nm | [74] |
2.1.1. Proteins
- Collagen
- Elastin
- Gelatin
2.1.2. Polysaccharides
- Hyaluronic acid
- Alginate
- Chitosan
2.2. Synthetic Hydrogels
| Hydrogel | Sensing | Analyte | Characteristics | Ref. |
|---|---|---|---|---|
| Electrochemical Methods | ||||
| Poly(vinyl alcohol), cellulose nanofibers and graphene | Electrochemical | Strain | Air Linear range: 0–500% strain Variations for light-emitting diode (LED) illumination vs. different resistance | [81] |
| TEMPO-oxidized cellulose in poly(acrylic acid) hydrogel, with ferric ions and polypyrrole | Amperometry | Mechanical change (strain) | Elongation ~890% Max storage modulus: 27.1 kPa Self-healing efficiencies (electrical and mechanical): ~99.4% electro-conductibility: ~3.9 S m−1. | [105] |
| Dendritic polyglycerol-poly(ethylene glycol) withaldehyde oxidoreductase | Amperometry | Benzaldehyde (BA) | LOD = 0.8 µM Linear range: 0.8–400 µM Max response at pH = 4.0 Signal of BA decreases with increase of pH | [141] |
| Optical Methods | ||||
| Polyacrylamide-phenylboronic acid | Surface plasmon resonance, Transmittance attenuation | Glucose | PBS buffer (pH = 7.4) LOD = 0.75 mM Linear range: 0–40 mM Sensitivity 0.05–0.13 dB/mM | [35] |
| Au nanoparticles-poly(ethylene glycol) diacrylate | Absorbance, Surface plasmon resonance, refractive index | Biotin | PBS buffer (pH = 7.4) Linear range: 25Μm–0.5 mM Sensitivity 70–110 nm/RIU Fluorescence λmax shift | [36] |
| Polyacrylamide-DNA hydrogel containing Au nanoparticles | Visual detection | Glucose | PBS buffer pH = 7.4 LOD = 0.44 mM Sensitivity: 1 mM Linear range: 0 to 15 mM glucose-boronic acid derivatives bind aptamer to disrupt the hydrogel, leading to the release of AuNPs | [37] |
| Supramolecular poly(ethylene glycol)-poly(ε-caprolactone) with CdTe quantum dots | Optical | pH, ions, biomolecules, chemicals, temperature | Emission of CdTe QD shifts from λem = 499 nm to λem = 549 nm | [39] |
| Poly(acrylic acid)-gum tragacanth nanoparticles with CdTe quantum dots (QDs) and glucose oxidize | Optical | Glucose | Enzyme-catalyzed oxidation of glucose produce H2O2 and quench fluorescence Linear range: 0–1 mM Blood samples media LOD-tunable | [40] |
| Sodium alginate, and poly(2-hydroxyethyl methacrylate) and poly(diallyldimethyl ammonium chloride) | Optical, Absorbance | pH | Water, acetic acid, sodium hydroxide Changing colors pH range 6.0–7.6 | [75] |
| Morpholino/oligonucleotide-polyacrylamide | Optical, volumetric | ssDNA | LOD = 10 pM, PBS buffer (pH = 7.4) Gel imaged using OnePlus 5t camera, Selective swelling caused by competitive displacement of morpholino crosslinks | [77] |
| Poly(N,N-dimethyl acrylamide–co-2-(dimethylmaleimido)-N-ethyl-acrylamide-co-vinyl-4,4-dimethylazlactone) (P(DMAAm-co-DMIAAm-co-VDMA) | Surface plasmon resonance | Lysophosphatidic acid (LPA) Cancer biomarker | LOD = 2 µM Linear range: 2–30 µM Selectivity in the presence of blood components (NaCl, urea, glucose, GPA, LPC) | [102] |
| Phenylboronic acid functionalized polyacrylamide | Optical | Glucose | Operating concentration range: 0–100 mM (in PBS, pH = 7.4) Linear range: 0–50 mM Sensitivity: 11.6 µW mM−1 pH operating range: 6–9 | [103] |
| Azlactone terpolymer P(DMAAm-co- DMIAAm-co-VDMA) | Surface plasmon resonance | Streptavidin | Linear range: 0.5–200 µM Monitoring of layer thickness of the hydrogel | [106] |
| Poly(ethylene glycol) diacrylate (PEGDA) | Fluorescence | mRNA, miRNA | LOD ≅ 6 amol (atto—10−18) (in vitro-transcribed model target). For quantification of full-length large mRNAs to small miRNAs | [108] |
| Poly(acrylic acid) with immobilized urease | Optical, volumetric | pH, urea | pH 2–12 range, 1.9–7.5 mM (urea), LOD = 40× mM (urea in blood) Change of volume and color | [140] |
| Poly(ethylene glycol) methacrylate, methyl methacrylate and maleimide | Fluorescence | Biotin-streptavidin (proteins pair model), DNA | Electrospunned nanofibers aligned into micropatterned array, that can be customized with probe that will interact with desired bioanalyte | [143] |
| Poly(acrylic acid-co-dimethylaminoethyl methacrylate) | pH-sensitive | Urea | LOD~1 mmol/L Linear range: 1–10 mmol/L PBS buffer pH = 7.4 Selectivity among urea, thiourea, N-methylurea and N,N,N′,N′-tetramethylurea | [144] |
| Poly(vinyl alcohol) with carboxyfluorescein and poly(methyl methacrylate-co-methacrylic acid) (Eudragit S100) | Optical | Urea | Infection-responsive coating for urinary catheters. pH > 7 dissolves the Eudragit layer, releasing the dye— visual change | [145] |
| Poly(acrylamide-co-acrylic acid) functionalized with urease | Particle spacing change, Debye diffraction measurement | Urea, urease inhibitor phenyl phosphorodiamidate (PPD) | LOD = 1 mM (urea) and 5.8 nM (PPD), both in water Linear range: 1–10 mM Selectivity in presence of formamide, N-methylurea, acetamide and N,N′-dimethylurea | [146] |
| Poly(N-isopropylacrylamide-co-2-acrylamido-2-methylpropane sulfonic acid) | Volumetric | Glucose | Operating concentration: 0–300 mg dL−1 | [147] |
3. Foldamers in Sensing
4. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Metkar, S.K.; Girigoswami, K. Diagnostic biosensors in medicine—A review. Biocatal. Agric. Biotechnol. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M.; Andronescu, E. Biosensors-on-Chip: An Up-to-Date Review. Molecules 2020, 25, 6013. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kim, H.; Hancke, G.P. Environmental monitoring systems: A review. IEEE Sens. J. 2013, 13, 1329–1339. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Yin, S.; Dong, J.; Kaynak, O. A Review on Soft Sensors for Monitoring, Control, and Optimization of Industrial Processes. IEEE Sens. J. 2021, 21, 12868–12881. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Chemical and Biological Sensors for Food-Quality Monitoring and Smart Packaging. Foods 2018, 7, 168. [Google Scholar] [CrossRef] [Green Version]
- Bergman, Å.; Heindel, J.J.; Jobling, S.; Zoeller, R.T.; Kidd, K.A.; Thomas, R. State of the Science of Endocrine Disrupting Chemicals 2012 Summary for Decision-Makers; United Nations Environment Programme: Nairobi, Kenya; World Health Organization: Geneva, Switzerland, 2013; pp. 4–7. ISBN 978-92-807-3274-0. [Google Scholar]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Okay, O. Hydrogel Sensors and Actuators; Gerlach, G., Arndt, K.-F., Eds.; Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2010; Volume 6, ISBN 978-3-540-75644-6. [Google Scholar]
- Cichosz, S.; Masek, A.; Zaborski, M. Polymer-based sensors: A review. Polym. Test. 2018, 67, 342–348. [Google Scholar] [CrossRef]
- Erol, O.; Pantula, A.; Liu, W.; Gracias, D.H. Transformer Hydrogels: A Review. Adv. Mater. Technol. 2019, 4, 1900043. [Google Scholar] [CrossRef] [Green Version]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Losi, V.; Magnaghi, L.R.; Biesuz, R. Current trends in polymer based sensors. Chemosensors 2021, 9, 108. [Google Scholar] [CrossRef]
- Krywko-Cendrowska, A.; Szweda, D.; Szweda, R. Well-defined conjugated macromolecules based on oligo(arylene ethynylene)s in sensing. Processes 2020, 8, 539. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.J.; Xu, F. Bioactuators based on stimulus-responsive hydrogels and their emerging biomedical applications. NPG Asia Mater. 2019, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Brighenti, R.; Li, Y.; Vernerey, F.J. Smart Polymers for Advanced Applications: A Mechanical Perspective Review. Front. Mater. 2020, 7, 196. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Wan, Y.; Carvalho, W.; Hu, L.; Serpe, M.J. Stimuli-Responsive Polymers for Sensing and Reacting to Environmental Conditions. Prog. Polym. Sci. 2021, 116, 101386. [Google Scholar] [CrossRef]
- Trzebicka, B.; Szweda, R.; Kosowski, D.; Szweda, D.; Otulakowski, Ł.; Haladjova, E.; Dworak, A. Thermoresponsive polymer-peptide/protein conjugates. Prog. Polym. Sci. 2017, 68, 35–76. [Google Scholar] [CrossRef]
- Shahrokhinia, A.; Biswas, P.; Reuther, J.F. Orthogonal synthesis and modification of polymer materials. J. Polym. Sci. 2021, 59, 1748–1786. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Pa’e, N.; Hashim, Z. Strategies in Improving Properties of Cellulose-Based Hydrogels for Smart Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 887–908. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Gnanou, Y.; Hadjichristidis, N.; Muthukumar, M.; Sheiko, S. Macromolecular Engineering: From Precise Synthesis to Macroscopic Materials and Applications; WILEY-VCH: Weinheim, Germany, 2022; ISBN 9783527344550. [Google Scholar]
- Sabbagh, F.; Muhamad, I.I.; Nazari, Z.; Mobini, P.; Mahmoudi Khatir, N. Investigation of acyclovir-loaded, acrylamide-based hydrogels for potential use as vaginal ring. Mater. Today Commun. 2018, 16, 274–280. [Google Scholar] [CrossRef]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Pinelli, F.; Magagnin, L.; Rossi, F. Progress in hydrogels for sensing applications: A review. Mater. Today Chem. 2020, 17, 100317. [Google Scholar] [CrossRef]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthc. Mater. 2021, 10, 2100062. [Google Scholar] [CrossRef]
- Sun, X.; Agate, S.; Salem, K.S.; Lucia, L.; Pal, L. Hydrogel-Based Sensor Networks: Compositions, Properties, and Applications—A Review. ACS Appl. Bio Mater. 2020, 4, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Tang, Y. Hydrogel based sensors for biomedical applications: An updated review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horkay, F.; Douglas, J.F. Polymer Gels: Basics, Challenges, and Perspectives. ACS Symp. Ser. 2018, 1296, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.; Yi, Y.; Lin, J.; Kong, F.; Chen, L.; Ni, Y.; Huang, L. Lignin reinforced hydrogels with fast self-recovery, multi-functionalities via calcium ion bridging for flexible smart sensing applications. Int. J. Biol. Macromol. 2022, 200, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Nandi, R.; Agam, Y.; Amdursky, N.; Nandi, R.; Agam, Y.; Amdursky, N. A Protein-Based Free-Standing Proton-Conducting Transparent Elastomer for Large-Scale Sensing Applications. Adv. Mater. 2021, 33, 2101208. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Wang, Y.; Li, W.; Zhao, W.; Duan, C.; Xiong, C.; Ni, Y. A green all-polysaccharide hydrogel platform for sensing and electricity harvesting/storage. J. Power Sources 2021, 493, 229711. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [Green Version]
- Temel, F.; Ozaytekin, I. The enhanced humidity sensing performance of calixarene/PMMA hybrid layers: QCM sensing mechanism. J. Mater. Sci. Mater. Electron. 2022, 1–15. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Zhang, W.; Dai, Y.; Xia, F. Gold nanoparticles-deranged double network for Janus adhesive-tough hydrogel as strain sensor. Chem. Eng. J. 2021, 420, 130447. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, B.; Du, Z.; Yang, C.; Kong, L.; Xu, L. Soft and plasmonic hydrogel optical probe for glucose monitoring. Nanophotonics 2021, 10, 3549–3558. [Google Scholar] [CrossRef]
- Miranda, B.; Moretta, R.; De Martino, S.; Dardano, P.; Rea, I.; Forestiere, C.; De Stefano, L. A PEGDA hydrogel nanocomposite to improve gold nanoparticles stability for novel plasmonic sensing platforms. J. Appl. Phys. 2021, 129, 033101. [Google Scholar] [CrossRef]
- Ma, Y.; Mao, Y.; An, Y.; Tian, T.; Zhang, H.; Yan, J.; Zhu, Z.; Yang, C.J. Target-responsive DNA hydrogel for non-enzymatic and visual detection of glucose. Analyst 2018, 143, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-B.; Yao, Y.; Montes-García, V.; Stoeckel, M.-A.; Von Holst, M.; Ciesielski, A.; Samorì, P.; Huang, C.; Yao, Y.; Montes-García, V.; et al. Highly Sensitive Strain Sensors Based on Molecules–Gold Nanoparticles Networks for High-Resolution Human Pulse Analysis. Small 2021, 17, 2007593. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ma, D.; Zhang, L.-M. Fabrication and properties of a supramolecular hybrid hydrogel doped with CdTe quantum dots. RSC Adv. 2015, 5, 58746–58754. [Google Scholar] [CrossRef]
- Qasemi, S.; Ghaemy, M. Highly sensitive and strongly fluorescent gum tragacanth based superabsorbent hydrogel as a new biosensor for glucose optical detection. J. Mater. Chem. C 2020, 8, 4148–4156. [Google Scholar] [CrossRef]
- Rinaldi, S. The diverse world of foldamers: Endless possibilities of self-assembly. Molecules 2020, 25, 3276. [Google Scholar] [CrossRef]
- Hill, D.J.; Mio, M.J.; Prince, R.B.; Hughes, T.S.; Moore, J.S. A Field Guide to Foldamers. Chem. Rev. 2001, 101, 3893–4011. [Google Scholar] [CrossRef]
- Mándity, I.M.; Fülöp, F. An overview of peptide and peptoid foldamers in medicinal chemistry. Expert Opin. Drug Discov. 2015, 10, 1163–1177. [Google Scholar] [CrossRef]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Yavuz, H.; Ünal, S.; Denizli, A. Molecularly Imprinted Polymer Based Sensors for Medical Applications. Sensors 2019, 19, 1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Advances in Molecularly Imprinted Polymers Based Affinity Sensors (Review). Polymers 2021, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, S.; Mishra, P. Molecularly imprinted polymer-based sensors for cancer biomarker detection. Sens. Actuators Rep. 2021, 3, 100061. [Google Scholar] [CrossRef]
- Herrera-Chacón, A.; Cetó, X.; del Valle, M. Molecularly imprinted polymers—Towards electrochemical sensors and electronic tongues. Anal. Bioanal. Chem. 2021, 413, 6117–6140. [Google Scholar] [CrossRef] [PubMed]
- Kuenzler, J.F. Hydrogels. In Encyclopedia of Polymer Science and Technology; Mark, H.F., Ed.; Wiley-Interscience: New York, NY, USA, 2004; pp. 691–722. [Google Scholar]
- Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to Hydrogels and Some Recent Applications. In Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Larsson, A.; Ekblad, T.; Andersson, O.; Liedberg, B. Photografted Poly(ethylene glycol) Matrix for Affinity Interaction Studies. Biomacromolecules 2006, 8, 287–295. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Estroff, L.A.; Hamilton, A.D. Water Gelation by Small Organic Molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Mateescu, A.; Wang, Y.; Dostalek, J.; Jonas, U. Thin hydrogel films for optical biosensor applications. Membranes 2012, 2, 49–69. [Google Scholar] [CrossRef] [Green Version]
- Jung, I.Y.; Kim, J.S.; Choi, B.R.; Lee, K.; Lee, H. Hydrogel Based Biosensors for In Vitro Diagnostics of Biochemicals, Proteins, and Genes. Adv. Healthc. Mater. 2017, 6, 1601475. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Echeverria, C.; Fernandes, S.N.; Godinho, M.H.; Borges, J.P.; Soares, P.I.P. Functional Stimuli-Responsive Gels: Hydrogels and Microgels. Gels 2018, 4, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.; Shi, S.; Liu, W.; Teng, H.; Piao, M. Processing and modification of hydrogel and its application in emerging contaminant adsorption and in catalyst immobilization: A review. Environ. Sci. Pollut. Res. 2020, 27, 12967–12994. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.Z.; Weng, Y.; Tan, H. Pyrophosphate ion-responsive alginate hydrogel as an effective fluorescent sensing platform for alkaline phosphatase detection. Chem. Commun. 2019, 55, 11450–11453. [Google Scholar] [CrossRef]
- Xiang, T.; Lu, T.; Zhao, W.F.; Zhao, C.S. Ionic-Strength Responsive Zwitterionic Copolymer Hydrogels with Tunable Swelling and Adsorption Behaviors. Langmuir 2019, 35, 1146–1155. [Google Scholar] [CrossRef]
- Hendi, A.; Hassan, M.U.; Elsherif, M.; Alqattan, B.; Park, S.; Yetisen, A.K.; Butt, H. Healthcare Applications of pH-Sensitive Hydrogel-Based Devices: A Review. Int. J. Nanomed. 2020, 15, 3887. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.F.; Adler, H.J.P. Review on Hydrogel-based pH Sensors and Microsensors. Sensors 2008, 8, 561. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef]
- Li, L.; Scheiger, J.M.; Levkin, P.A.; Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, R.V.; Biswanath, S. Electrically responsive smart hydrogels in drug delivery: A review. J. Appl. Biomater. Biomech. 2007, 5, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Yu, A.; Lai, G. Conductive hydrogel-based electrochemical sensor: A soft platform for capturing analyte. Chemosensors 2021, 9, 282. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Chen, C.; Cheng, Y. Magnetic-responsive hydrogels: From strategic design to biomedical applications. J. Control. Release 2021, 335, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Q.; Du, J. Recent advances in magnetic hydrogels. Polym. Int. 2016, 65, 1365–1372. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2016, 8, 127–143. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, J.; Jabbari, E.; Khosroshahi, M.E.; Boroujerdi, M. Swelling characterization of anionic acrylic acid hydrogel in an external electric field. Iran. Polym. J. Engl. Ed. 2006, 15, 891–900. [Google Scholar]
- Wei, X.; Tian, T.; Jia, S.; Zhu, Z.; Ma, Y.; Sun, J.; Lin, Z.; Yang, C.J. Microfluidic Distance Readout Sweet Hydrogel Integrated Paper-Based Analytical Device (μDiSH-PAD) for Visual Quantitative Point-of-Care Testing. Anal. Chem. 2016, 88, 2345–2352. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L. Preparation of a visual pH-sensing film based on tara gum incorporating cellulose and extracts from grape skins. Sens. Actuators B Chem. 2016, 235, 401–407. [Google Scholar] [CrossRef]
- Kertkal, N.; Jinawong, P.; Rithiyong, A.; Kusuktham, B. Hybrid Hydrogels for pH Indicator. Silicon 2021. [Google Scholar] [CrossRef]
- Aronoff, M.R.; Hiebert, P.; Hentzen, N.B.; Werner, S.; Wennemers, H. Imaging and targeting LOX-mediated tissue remodeling with a reactive collagen peptide. Nat. Chem. Biol. 2021, 17, 865–871. [Google Scholar] [CrossRef]
- Langford, G.J.; Raeburn, J.; Ferrier, D.C.; Hands, P.J.W.; Shaver, M.P. Morpholino Oligonucleotide Cross-Linked Hydrogels as Portable Optical Oligonucleotide Biosensors. ACS Sens. 2019, 4, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, N.; Saha, B.; De, P. Recent progress in polymer-based optical chemosensors for Cu2+ and Hg2+ Ions: A comprehensive review. Eur. Polym. J. 2021, 145, 110233. [Google Scholar] [CrossRef]
- Dowlatshahi, S.; Abdekhodaie, M.J. Electrochemical prostate-specific antigen biosensors based on electroconductive nanomaterials and polymers. Clin. Chim. Acta. 2021, 516, 111–135. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Martinez, J.G.; Jager, E.W.H.; Phopase, J.; Turner, A.P.F. Type i Collagen-Derived Injectable Conductive Hydrogel Scaffolds as Glucose Sensors. ACS Appl. Mater. Interfaces 2018, 10, 16244–16249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, C.; Yue, Y.; Gan, L.; Xu, X.; Mei, C.; Han, J. Highly stretchable and self-healing strain sensors based on nanocellulose-supported graphene dispersed in electro-conductive hydrogels. Nanomaterials 2019, 9, 937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Wang, A.; Lyv, F.; Lai, G.; Zhang, H.; Yu, J.; Lin, C.T.; Yu, A.; Su, W. Electrochemical antioxidant screening based on a chitosan hydrogel. Bioelectrochemistry 2018, 121, 7–10. [Google Scholar] [CrossRef]
- Wei, B.; Zhong, H.; Wang, L.; Liu, Y.; Xu, Y.; Zhang, J.; Xu, C.; He, L.; Wang, H. Facile preparation of a collagen-graphene oxide composite: A sensitive and robust electrochemical aptasensor for determining dopamine in biological samples. Int. J. Biol. Macromol. 2019, 135, 400–406. [Google Scholar] [CrossRef]
- Bandiera, A.; Corich, L.; Tommasi, S.; De Bortoli, M.; Pelizzo, P.; Stebel, M.; Paladin, D.; Passamonti, S. Human elastin-like polypeptides as a versatile platform for exploitation of ultrasensitive bilirubin detection by UnaG. Biotechnol. Bioeng. 2020, 117, 354–361. [Google Scholar] [CrossRef]
- Ibáñez-Fonseca, A.; Alonso, M.; Arias, F.J.; Rodríguez-Cabello, J.C. Förster Resonance Energy Transfer-Paired Hydrogel Forming Silk-Elastin-Like Recombinamers by Recombinant Conjugation of Fluorescent Proteins. Bioconjug. Chem. 2017, 28, 828–835. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, S.; Chen, Y.; Ling, H.; Zhao, L.; Luo, G.; Wang, X.; Hartel, M.C.; Liu, H.; Xue, Y.; et al. Gelatin Methacryloyl-Based Tactile Sensors for Medical Wearables. Adv. Funct. Mater. 2020, 30, 2003601. [Google Scholar] [CrossRef]
- Wang, J.; Tang, F.; Wang, Y.; Lu, Q.; Liu, S.; Li, L. Self-Healing and Highly Stretchable Gelatin Hydrogel for Self-Powered Strain Sensor. ACS Appl. Mater. Interfaces 2020, 12, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.K.; Dule, M.; Paul, R.; Dash, J.; Anas, M.; Mandal, T.K.; Das, P.; Das, N.C.; Banerjee, S. Carbon Dot Cross-Linked Gelatin Nanocomposite Hydrogel for pH-Sensing and pH-Responsive Drug Delivery. ACS Biomater. Sci. Eng. 2020, 6, 5662–5674. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, Y.; Yin, Z.; Cai, X.; Xia, X.; Donovan, M.J.; Chen, L.; Chen, Z.; Tan, W. In Situ Gastric pH Imaging with Hydrogel Capsule Isolated Paramagnetic Metallo-albumin Complexes. Anal. Chem. 2021, 93, 5939–5946. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Sun, J.; Chen, M.; Tian, J.; Yin, H.; Yin, J. A hyaluronic acid fluorescent hydrogel based on fluorescence resonance energy transfer for sensitive detection of hyaluronidase. Anal. Bioanal. Chem. 2020, 412, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Gao, C.; Shen, L.; Qu, C.; Zhang, X.; Yang, L.; Feng, Q.; Tang, R. Alginate hydrogel-embedded capillary sensor for quantitative immunoassay with naked eye. Sensors 2020, 20, 4831. [Google Scholar] [CrossRef] [PubMed]
- Bornhoeft, L.R.; Biswas, A.; McShane, M.J. Composite hydrogels with engineered microdomains for optical glucose sensing at low oxygen conditions. Biosensors 2017, 7, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buk, V.; Emregul, E.; Emregul, K.C. Alginate copper oxide nano-biocomposite as a novel material for amperometric glucose biosensing. Mater. Sci. Eng. C 2017, 74, 307–314. [Google Scholar] [CrossRef]
- Tamayol, A.; Akbari, M.; Zilberman, Y.; Comotto, M.; Lesha, E.; Serex, L.; Bagherifard, S.; Chen, Y.; Fu, G.; Ameri, S.K.; et al. Flexible pH-Sensing Hydrogel Fibers for Epidermal Applications. Adv. Healthc. Mater. 2016, 5, 711–719. [Google Scholar] [CrossRef] [Green Version]
- Gunatilake, U.B.; Garcia-Rey, S.; Ojeda, E.; Basabe-Desmonts, L.; Benito-Lopez, F. TiO2Nanotubes Alginate Hydrogel Scaffold for Rapid Sensing of Sweat Biomarkers: Lactate and Glucose. ACS Appl. Mater. Interfaces 2021, 13, 37734–37745. [Google Scholar] [CrossRef]
- Xiong, S.; Marin, L.; Duan, L.; Cheng, X. Fluorescent chitosan hydrogel for highly and selectively sensing of p-nitrophenol and 2,4,6-trinitrophenol. Carbohydr. Polym. 2019, 225, 115253. [Google Scholar] [CrossRef]
- Conghaile, P.; Kumar, R.; Ferrer, M.L.; Leech, D. Glucose oxidation by enzyme electrodes using genipin to crosslink chitosan, glucose oxidase and amine-containing osmium redox complexes. Electrochem. Commun. 2020, 113, 106703. [Google Scholar] [CrossRef]
- Zanini, V.P.; De Mishima, B.L.; Solís, V. An amperometric biosensor based on lactate oxidase immobilized in laponite–chitosan hydrogel on a glassy carbon electrode. Application to the analysis of l-lactate in food samples. Sens. Actuators B Chem. 2011, 155, 75–80. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, J.; Wu, C.; Hu, X.; Lu, Y.; Wang, G.; Yu, F.; Zhang, X.; Wang, Y. Flexible and self-healing electrochemical hydrogel sensor with high efficiency toward glucose monitoring. Biosens. Bioelectron. 2020, 155, 112105. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Cao, Z.; Xie, Q.; Fu, Y.; Tan, Y.; Ma, M.; Yao, S. One-pot electrodeposition of 3-aminopropyltriethoxysilane–chitosan hybrid gel film to immobilize glucose oxidase for biosensing. Sens. Actuators B Chem. 2011, 157, 282–289. [Google Scholar] [CrossRef]
- Shukla, S.K.; Lavon, A.; Shmulevich, O.; Ben-Yoav, H. The effect of loading carbon nanotubes onto chitosan films on electrochemical dopamine sensing in the presence of biological interference. Talanta 2018, 181, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ji, C.; Lü, B.; Rodin, M.; Paradies, J.; Yin, M.; Kuckling, D. Dually Crosslinked Supramolecular Hydrogel for Cancer Biomarker Sensing. ACS Appl. Mater. Interfaces 2020, 12, 36873–36881. [Google Scholar] [CrossRef]
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Glucose Sensing with Phenylboronic Acid Functionalized Hydrogel-Based Optical Diffusers. ACS Nano 2018, 12, 2283–2291. [Google Scholar] [CrossRef]
- Mao, X.; Pan, S.; Zhou, D.; He, X.; Zhang, Y. Fabrication of DNAzyme-functionalized hydrogel and its application for visible detection of circulating tumor DNA. Sens. Actuators B Chem. 2019, 285, 385–390. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, K.; Song, Y.; Han, J.; Yue, Y.; Biswas, S.K.; Wu, Q.; Xiao, H. A skin-inspired stretchable, self-healing and electro-conductive hydrogel with a synergistic triple network for wearable strain sensors applied in human-motion detection. Nanomaterials 2019, 9, 1737. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yu, X.; Herberg, A.; Kuckling, D. Biomolecule Sensor Based on Azlactone-Modified Hydrogel Films. Macromol. Rapid Commun. 2019, 40, 1800674. [Google Scholar] [CrossRef]
- Chu, T.W.; Feng, J.; Yang, J.; Kopeček, J. Hybrid polymeric hydrogels via peptide nucleic acid (PNA)/DNA complexation. J. Control. Release 2015, 220, 608–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, N.W.; Kim, J.; Chapin, S.C.; Duong, T.; Donohue, E.; Pandey, P.; Broom, W.; Hill, W.A.; Doyle, P.S. Multiplexed detection of mRNA using porosity-tuned hydrogel microparticles. Anal. Chem. 2012, 84, 9370–9378. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokulnathan, T.; Ramaraj, S.; Chen, S.-M.; Han-Yu, Y. Eco-Friendly Synthesis of Biocompatible Pectin Stabilized Graphene Nanosheets Hydrogel and Their Application for the Simultaneous Electrochemical Determination of Dopamine and Paracetamol in Real Samples. J. Electrochem. Soc. 2018, 165, B240–B249. [Google Scholar] [CrossRef]
- Vashist, S.K.; Luong, J.H.T. Bioanalytical Requirements and Regulatory Guidelines for Immunoassays. In Handbook of Immunoassay Technologies: Approaches, Performances, and Applications; Academic Press: Cambridge, MA, USA, 2018; pp. 81–95. [Google Scholar] [CrossRef]
- Di Lullo, G.A.; Sweeney, S.M.; Körkkö, J.; Ala-Kokko, L.; San Antonio, J.D. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J. Biol. Chem. 2002, 277, 4223–4231. [Google Scholar] [CrossRef] [Green Version]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Terzi, A.; Sannino, A.; Madaghiele, M. Mimicking the Hierarchical Organization of Natural Collagen: Toward the Development of Ideal Scaffolding Material for Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 258. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, C.; Luo, X.; Wang, X.; Jiang, H. Recent advances of collagen-based biomaterials: Multi-hierarchical structure, modification and biomedical applications. Mater. Sci. Eng. C 2019, 99, 1509–1522. [Google Scholar] [CrossRef]
- Kagan, H.M.; Li, W. Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. J. Cell. Biochem. 2003, 88, 660–672. [Google Scholar] [CrossRef]
- Levene, C.I.; Gross, J. Alterations in state of molecular aggregation of collagen induced in chick embryos by beta-aminopropionitrile (lathyrus factor). J. Exp. Med. 1959, 110, 771–790. [Google Scholar] [CrossRef] [Green Version]
- Vallet, S.D.; Ricard-Blum, S. Lysyl oxidases: From enzyme activity to extracellular matrix cross-links. Essays Biochem. 2019, 63, 349–364. [Google Scholar] [CrossRef]
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; de Carvalho, C.M. Elastin structure and its involvement in skin photoageing. Int. J. Cosmet. Sci. 2017, 39, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.B. Food Science. Nutr. Today 1998, 33, 106–112. [Google Scholar] [CrossRef]
- Sanderson, G. Thickening and gelling agents for food. Trends Food Sci. Technol. 1993, 4, 233–234. [Google Scholar] [CrossRef]
- Köwitsch, A.; Zhou, G.; Groth, T. Medical application of glycosaminoglycans: A review. J. Tissue Eng. Regen. Med. 2018, 12, e23–e41. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Gao, Y.; Chen, G.; Huang, X.; Xu, X.; Lv, J.; Wang, J.; Xu, D.; Liu, G. Recent Advances and Future Trends in the Detection of Contaminants by Molecularly Imprinted Polymers in Food Samples. Front. Chem. 2020, 8, 1142. [Google Scholar] [CrossRef]
- Moghaddam, M.K.; Mortazavi, S.M.; Khayamian, T. Preparation of calcium alginate microcapsules containing n-nonadecane by a melt coaxial electrospray method. J. Electrostat. 2015, 73, 56–64. [Google Scholar] [CrossRef]
- Tai, Y.; Mulle, M.; Ventura, I.A.; Lubineau, G. A highly sensitive, low-cost, wearable pressure sensor based on conductive hydrogel spheres. Nanoscale 2015, 7, 14766–14773. [Google Scholar] [CrossRef]
- Ellis, M.E. Alkaline Phosphatase Level Test. Available online: http://www.healthline.com/health/alp#Overview1 (accessed on 5 November 2021).
- Jayakumar, A.; Jose, V.K.; Lee, J.M. Hydrogels for Medical and Environmental Applications. Small Methods 2020, 4, 177–178. [Google Scholar] [CrossRef]
- Blackman, L.D.; Gunatillake, P.A.; Cass, P.; Locock, K.E.S. An introduction to zwitterionic polymer behavior and applications in solution and at surfaces. Chem. Soc. Rev. 2019, 48, 757–770. [Google Scholar] [CrossRef]
- Adamus, A.; Komasa, J.; Kadłubowski, S.; Ulański, P.; Rosiak, J.M.; Kawecki, M.; Klama-Baryła, A.; Dworak, A.; Trzebicka, B.; Szweda, R. Thermoresponsive poly[tri(ethylene glycol) monoethyl ether methacrylate]-peptide surfaces obtained by radiation grafting-synthesis and characterisation. Colloids Surf. B Biointerfaces 2016, 145, 185–193. [Google Scholar] [CrossRef]
- Dohi, S.; Suzuki, Y.; Matsumoto, A. One-shot radical polymerization of vinyl monomers with different reactivity accompanying spontaneous delay of polymerization for the synthesis of double-network hydrogels. Polym. Int. 2020, 69, 954–963. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Click Chemistry-Based Injectable Hydrogels and Bioprinting Inks for Tissue Engineering Applications. Tissue Eng. Regen. Med. 2018, 15, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Han, Y.; Sun, H.Y.; Hilborn, J.; Shi, L. Click chemistry-based biopolymeric hydrogels for regenerative medicine. Biomed. Mater. 2021, 16, 022003. [Google Scholar] [CrossRef] [PubMed]
- Dworak, A.; Lipowska, D.; Szweda, D.; Suwinski, J.; Trzebicka, B.; Szweda, R. Degradable polymeric nanoparticles by aggregation of thermoresponsive polymers and “click” chemistry. Nanoscale 2015, 7, 16823–16833. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Liu, Y.; Hsu, S.H. Hydrogels based on schiff base linkages for biomedical applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhang, Y.; Liu, W. Bioinspired fabrication of high strength hydrogels from non-covalent interactions. Prog. Polym. Sci. 2017, 71, 1–25. [Google Scholar] [CrossRef]
- Shang, J.; Theato, P. Smart composite hydrogel with pH-, ionic strength- and temperature-induced actuation. Soft Matter 2018, 14, 8401–8407. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, H.; Yang, Z.; Xu, B. Supramolecular Hydrogels Respond to Ligand-Receptor Interaction. J. Am. Chem. Soc. 2003, 125, 13680–13681. [Google Scholar] [CrossRef]
- Fan, H.; Wang, L.; Feng, X.; Bu, Y.; Wu, D.; Jin, Z. Supramolecular Hydrogel Formation Based on Tannic Acid. Macromolecules 2017, 50, 666–676. [Google Scholar] [CrossRef]
- Haburcak, R.; Shi, J.; Du, X.; Yuan, D.; Xu, B. Ligand-Receptor Interaction Modulates the Energy Landscape of Enzyme-Instructed Self-Assembly of Small Molecules. J. Am. Chem. Soc. 2016, 138, 15397–15404. [Google Scholar] [CrossRef]
- Vancoillie, G.; Hoogenboom, R.; Sparacino, G.; Facchinetti, A.; Devries, J.H. Responsive Boronic Acid-Decorated (Co)polymers: From Glucose Sensors to Autonomous Drug Delivery. Sensors 2016, 16, 1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, K.G.; Park, S.Y. Biosensor Array of Interpenetrating Polymer Network with Photonic Film Templated from Reactive Cholesteric Liquid Crystal and Enzyme-Immobilized Hydrogel Polymer. Adv. Funct. Mater. 2018, 28, 1707562. [Google Scholar] [CrossRef]
- Dey, P.; Adamovski, M.; Friebe, S.; Badalyan, A.; Mutihac, R.C.; Paulus, F.; Leimkühler, S.; Wollenberger, U.; Haag, R. Dendritic polyglycerol-poly(ethylene glycol)-based polymer networks for biosensing application. ACS Appl. Mater. Interfaces 2014, 6, 8937–8941. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, Y.; Ran, F.; Li, C.; Dai, L.; Li, H.; Yu, F.; Zheng, C.; Si, C. Lignin nanoparticles for hydrogel-based pressure sensor. Ind. Crops Prod. 2022, 176, 114366. [Google Scholar] [CrossRef]
- Kalaoglu-Altan, O.I.; Sanyal, R.; Sanyal, A. Micropatterned Reactive Nanofibers: Facile Fabrication of a Versatile Biofunctionalizable Interface. ACS Appl. Polym. Mater. 2020, 2, 4026–4036. [Google Scholar] [CrossRef]
- Erfkamp, J.; Guenther, M.; Gerlach, G. Enzyme-Functionalized Piezoresistive Hydrogel Biosensors for the Detection of Urea. Sensors 2019, 19, 2858. [Google Scholar] [CrossRef] [Green Version]
- Milo, S.; Thet, N.T.; Liu, D.; Nzakizwanayo, J.; Jones, B.V.; Jenkins, A.T.A. An in-situ infection detection sensor coating for urinary catheters. Biosens. Bioelectron. 2016, 81, 166–172. [Google Scholar] [CrossRef]
- Li, G.; Xiao, F.; Liao, S.; Chen, Q.; Zhou, J.; Wu, Z.; Yu, R. Label-free 2D colloidal photonic crystal hydrogel biosensor for urea and urease inhibitor. Sens. Actuators B Chem. 2018, 277, 591–597. [Google Scholar] [CrossRef]
- Fei, R.; Means, A.K.; Abraham, A.A.; Locke, A.K.; Coté, G.L.; Grunlan, M.A. Self-Cleaning, Thermoresponsive P(NIPAAm-co-AMPS) Double Network Membranes for Implanted Glucose Biosensors. Macromol. Mater. Eng. 2016, 301, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-J.; Pruzinsky, S.A.; Braun, P.V. Glucose-Sensitive Inverse Opal Hydrogels: Analysis of Optical Diffraction Response. Langmuir 2004, 20, 3096–3106. [Google Scholar] [CrossRef]
- Alexeev, V.L.; Sharma, A.C.; Goponenko, A.V.; Das, S.; Lednev, I.K.; Wilcox, C.S.; Finegold, D.N.; Asher, S.A. High Ionic Strength Glucose-Sensing Photonic Crystal. Anal. Chem. 2003, 75, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- Guichard, G.; Huc, I. Synthetic foldamers. Chem. Commun. 2011, 47, 5933–5941. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.; Huc, I. Foldamers: Structure, Properties, and Applications. In Foldamers: Structure, Properties, and Applications; Hecht, S., Huc, I., Eds.; Wiley: Hoboken, NJ, USA, 2007; pp. 1–434. ISBN 9783527315635. [Google Scholar]
- Ożga, K.; Drewniak-Świtalska, M.; Rudzińska-Szostak, E.; Berlicki, Ł. Towards Foldameric Miniproteins: A Helix-Turn-Helix Motif. Chempluschem 2021, 86, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Huc, I.; Kwon, S.; Lee, H.S. Synthetic Foldamers: Rational Design of Advanced Structures with Diverse Applications. Chempluschem 2021, 86, 1042–1043. [Google Scholar] [CrossRef]
- Nanjan, P.; Porel, M. Sequence-defined non-natural polymers: Synthesis and applications. Polym. Chem. 2019, 10, 5406–5424. [Google Scholar] [CrossRef]
- Solleder, S.C.; Schneider, R.V.; Wetzel, K.S.; Boukis, A.C.; Meier, M.A.R. Recent Progress in the Design of Monodisperse, Sequence-Defined Macromolecules. Macromol. Rapid Commun. 2017, 38, 1600711. [Google Scholar] [CrossRef]
- Nerantzaki, M.; Lutz, J.-F. Multistep Growth “Polymerizations”. Macromol. Chem. Phys. 2021, 2100368. [Google Scholar] [CrossRef]
- Yang, C.; Wu, K.B.; Deng, Y.; Yuan, J.; Niu, J. Geared Toward Applications: A Perspective on Functional Sequence-Controlled Polymers. ACS Macro Lett. 2021, 10, 243–257. [Google Scholar] [CrossRef]
- Aksakal, R.; Mertens, C.; Soete, M.; Badi, N.; Du Prez, F. Applications of Discrete Synthetic Macromolecules in Life and Materials Science: Recent and Future Trends. Adv. Sci. 2021, 8, 2004038. [Google Scholar] [CrossRef]
- Hill, S.A.; Steinfort, R.; Hartmann, L. Progress, challenges and future directions of heterocycles as building blocks in iterative methodologies towards sequence-defined oligomers and polymers. Polym. Chem. 2021, 12, 4439–4450. [Google Scholar] [CrossRef]
- Deng, Z.; Shi, Q.; Tan, J.; Hu, J.; Liu, S. Sequence-Defined Synthetic Polymers for New-Generation Functional Biomaterials. ACS Mater. Lett. 2021, 3, 1339–1356. [Google Scholar] [CrossRef]
- Li, Z.; Cai, B.; Yang, W.; Chen, C.L. Hierarchical Nanomaterials Assembled from Peptoids and Other Sequence-Defined Synthetic Polymers. Chem. Rev. 2021, 121, 14031–14087. [Google Scholar] [CrossRef] [PubMed]
- Khoury, L.R.; Popa, I. Chemical unfolding of protein domains induces shape change in programmed protein hydrogels. Nat. Commun. 2019, 10, 5439. [Google Scholar] [CrossRef] [PubMed]
- Pasco, M.; Dolain, C.; Guichard, G. Foldamers in Medicinal Chemistry. Compr. Supramol. Chem. II 2017, 5, 89. [Google Scholar] [CrossRef] [Green Version]
- Godballe, T.; Nilsson, L.L.; Petersen, P.D.; Jenssen, H. Antimicrobial β-Peptides and α-Peptoids. Chem. Biol. Drug Des. 2011, 77, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Seebach, D.; Hook, D.F.; Glättli, A. Helices and other secondary structures of β- and γ-peptides. Biopolym.-Pept. Sci. Sect. 2006, 84, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Mateus, P.; Chandramouli, N.; Mackereth, C.D.; Kauffmann, B.; Ferrand, Y.; Huc, I. Allosteric Recognition of Homomeric and Heteromeric Pairs of Monosaccharides by a Foldamer Capsule. Angew. Chemie 2020, 132, 5846–5854. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wicher, B.; MØndez-Ardoy, A.; Li, X.; Pecastaings, G.; Buffeteau, T.; Bassani, D.M.; Maurizot, V.; Huc, I.; Wicher, B.; et al. Loading Linear Arrays of Cu II Inside Aromatic Amide Helices. Angew. Chem. Int. Ed. 2021, 60, 18461–18466. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, C.; Zhan, Y.; Deng, X.; Wang, Y.; Jiang, H. Locking Interconversion of Aromatic Oligoamide Foldamers by Intramolecular Side-chain Crosslinking: Toward Absolute Control of Helicity in Synthetic Aromatic Foldamers. Chem.—Eur. J. 2017, 23, 5361–5367. [Google Scholar] [CrossRef]
- Zhang, D.W.; Zhao, X.; Hou, J.L.; Li, Z.T. Aromatic Amide Foldamers: Structures, Properties, and Functions. Chem. Rev. 2012, 112, 5271–5316. [Google Scholar] [CrossRef]
- Laursen, J.S.; Engel-Andreasen, J.; Olsen, C.A. β-Peptoid Foldamers at Last. Acc. Chem. Res. 2015, 48, 2696–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connolly, M.D.; Xuan, S.; Molchanova, N.; Zuckermann, R.N. Submonomer synthesis of sequence defined peptoids with diverse side-chains. Methods Enzymol. 2021, 656, 241–270. [Google Scholar] [CrossRef] [PubMed]
- Bécart, D.; Diemer, V.; Salaün, A.; Oiarbide, M.; Nelli, Y.R.; Kauffmann, B.; Fischer, L.; Palomo, C.; Guichard, G. Helical Oligourea Foldamers as Powerful Hydrogen Bonding Catalysts for Enantioselective C-C Bond-Forming Reactions. J. Am. Chem. Soc. 2017, 139, 12524–12532. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, C.F. Helicenes: Synthesis and Applications. Chem. Rev. 2011, 112, 1463–1535. [Google Scholar] [CrossRef]
- Shankar, S.; Rahim, J.U.; Rai, R. Self-Assembly in Peptides Containing β-and γ-amino Acids. Curr. Protein Pept. Sci. 2020, 21, 584–597. [Google Scholar] [CrossRef]
- Misra, R.; Sharma, A.; Shiras, A.; Gopi, H.N. Backbone Engineered γ-Peptide Amphitropic Gels for Immobilization of Semiconductor Quantum Dots and 2D Cell Culture. Langmuir 2017, 33, 7762–7768. [Google Scholar] [CrossRef]
- Hegedüs, Z.; Martinek, T.A. Molecular Recognition Using Foldamers. Compr. Supramol. Chem. 2017, 4, 511–537. [Google Scholar] [CrossRef]
- Kirshenbaum, K. Foldamers: Structure, Properties, and Applications. Edited by Stefan Hecht and Ivan Huc. ChemBioChem 2008, 9, 157–158. [Google Scholar] [CrossRef]
- Juwarker, H.; Suk, J.M.; Jeong, K.S. Foldamers with helical cavities for binding complementary guests. Chem. Soc. Rev. 2009, 38, 3316–3325. [Google Scholar] [CrossRef]
- Mateus, P.; Wicher, B.; Ferrand, Y.; Huc, I. Alkali and alkaline earth metal ion binding by a foldamer capsule: Selective recognition of magnesium hydrate. Chem. Commun. 2017, 53, 9300–9303. [Google Scholar] [CrossRef] [Green Version]
- Prince, R.B.; Okada, T.; Moore, J.S. Controlling the secondary structure of nonbiological oligomers with solvophobic and coordination interactions. Angew. Chem.-Int. Ed. 1999, 38, 233–236. [Google Scholar] [CrossRef]
- Petitjean, A.; Cuccia, L.A.; Lehn, J.M.; Nierengarten, H.; Schmutz, M. Cation-promoted hierarchical formation of supramolecular assemblies of self-organized helical molecular components. Angew. Chem.-Int. Ed. 2002, 41, 1195–1198. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhong, Z. Tuning the sensitivity of a foldamer-based mercury sensor by its folding energy. J. Am. Chem. Soc. 2006, 128, 9988–9989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamato, K.; Yuan, L.; Feng, W.; Helsel, A.J.; Sanford, A.R.; Zhu, J.; Deng, J.; Zeng, X.C.; Gong, B. Crescent oligoamides as hosts: Conformation-dependent binding specificity. Org. Biomol. Chem. 2009, 7, 3643–3647. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ren, C.; Zeng, H. Surprisingly High Selectivity and High Affinity in Mercury Recognition by H-Bonded Cavity-Containing Aromatic Foldarands. J. Am. Chem. Soc. 2017, 139, 5387–5396. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; Qi, S.; Deng, X.; Yang, B.; Liu, J.; Dong, Z. A Switchable Helical Capsule for Encapsulation and Release of Potassium Ion. J. Org. Chem. 2018, 83, 1898–1902. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Z.; Zhang, L.; Wang, H.; Zhang, D.; Hou, J.; Li, Z. Aromatic Amide Polymers that Form Two Helical Conformations with Twist Sense Bias in Water. Chin. J. Chem. 2016, 34, 678–682. [Google Scholar] [CrossRef]
- Chang, K.J.; Kang, B.N.; Lee, M.H.; Jeong, K.S. Oligoindole-based foldamers with a helical conformation induced by chloride. J. Am. Chem. Soc. 2005, 127, 12214–12215. [Google Scholar] [CrossRef]
- Meudtner, R.M.; Hecht, S. Helicity Inversion in Responsive Foldamers Induced by Achiral Halide ion Guests. Angew. Chem.-Int. Ed. 2008, 47, 4926–4930. [Google Scholar] [CrossRef]
- Juwarker, H.; Lenhardt, J.M.; Pham, D.M.; Craig, S.L. 1,2,3-Triazole CH⋅⋅⋅Cl− Contacts Guide Anion Binding and Concomitant Folding in 1,4-Diaryl Triazole Oligomers. Angew. Chem.-Int. Ed. 2008, 47, 3740–3743. [Google Scholar] [CrossRef]
- Wang, Y.; Bie, F.; Jiang, H. Controlling binding affinities for anions by a photoswitchable foldamer. Org. Lett. 2010, 12, 3630–3633. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.M.; Naidu, V.R.; Liu, X.; Lah, M.S.; Jeong, K.S. A foldamer-based chiroptical molecular switch that displays complete inversion of the helical sense upon anion binding. J. Am. Chem. Soc. 2011, 133, 13938–13941. [Google Scholar] [CrossRef] [PubMed]
- Chandramouli, N.; Ferrand, Y.; Kauffmann, B.; Huc, I. Citric acid encapsulation by a double helical foldamer in competitive solvents. Chem. Commun. 2016, 52, 3939–3942. [Google Scholar] [CrossRef] [PubMed]
- Tanatani, A.; Mio, M.J.; Moore, J.S. Chain length-dependent affinity of helical foldamers for a rodlike guest. J. Am. Chem. Soc. 2001, 123, 1792–1793. [Google Scholar] [CrossRef]
- Li, C.; Wang, G.T.; Yi, H.P.; Jiang, X.K.; Li, Z.T.; Wang, R.X. Diastereomeric recognition of chiral foldamer receptors for chiral glucoses. Org. Lett. 2007, 9, 1797–1800. [Google Scholar] [CrossRef]
- Waki, M.; Abe, H.; Inouye, M. Translation of Mutarotation into Induced Circular Dichroism Signals through Helix Inversion of Host Polymers. Angew. Chem.-Int. Ed. 2007, 46, 3059–3061. [Google Scholar] [CrossRef]
- Jeon, H.G.; Jung, J.Y.; Kang, P.; Choi, M.G.; Jeong, K.S. Folding-Generated Molecular Tubes Containing One-Dimensional Water Chains. J. Am. Chem. Soc. 2016, 138, 92–95. [Google Scholar] [CrossRef]
- Takashima, S.; Abe, H.; Inouye, M. Copper(II)/phenanthroline-mediated CD-enhancement and chiral memory effect on a meta-ethynylpyridine oligomer. Chem. Commun. 2012, 48, 3330–3332. [Google Scholar] [CrossRef]
- Chandramouli, N.; Ferrand, Y.; Lautrette, G.; Kauffmann, B.; MacKereth, C.D.; Laguerre, M.; Dubreuil, D.; Huc, I. Iterative design of a helically folded aromatic oligoamide sequence for the selective encapsulation of fructose. Nat. Chem. 2015, 7, 334–341. [Google Scholar] [CrossRef]
- Lautrette, G.; Wicher, B.; Kauffmann, B.; Ferrand, Y.; Huc, I. Iterative Evolution of an Abiotic Foldamer Sequence for the Recognition of Guest Molecules with Atomic Precision. J. Am. Chem. Soc. 2016, 138, 10314–10322. [Google Scholar] [CrossRef]
- Ferrand, Y.; Kendhale, A.M.; Kauffmann, B.; Grélard, A.; Marie, C.; Blot, V.; Pipelier, M.; Dubreuil, D.; Huc, I. Diastereoselective encapsulation of tartaric acid by a helical aromatic oligoamide. J. Am. Chem. Soc. 2010, 132, 7858–7859. [Google Scholar] [CrossRef] [PubMed]
- Mateus, P.; Wicher, B.; Ferrand, Y.; Huc, I. Carbohydrate binding through first- and second-sphere coordination within aromatic oligoamide metallofoldamers. Chem. Commun. 2018, 54, 5078–5081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishinaga, T.; Tanatani, A.; Oh, K.; Moore, J.S. The size-selective synthesis of folded oligomers by dynamic templation. J. Am. Chem. Soc. 2002, 124, 5934–5935. [Google Scholar] [CrossRef] [PubMed]
- Amat, M.; Cantó, M.; Llor, N.; Ponzo, V.; Pérez, M.; Bosch, J. Dynamic kinetic resolution and desymmetrization of enantiotopic groups by cyclodehydration of racemic or prochiral δ-oxoesters with (R)-phenylglycinol: Enantioselective synthesis of piperidines. Angew. Chem.-Int. Ed. 2002, 41, 335–338. [Google Scholar] [CrossRef]
- Gan, Q.; Ferrand, Y.; Bao, C.; Kauffmann, B.; Grélard, A.; Jiang, H.; Huc, I. Helix-rod host-guest complexes with shuttling rates much faster than disassembly. Science 2011, 331, 1172–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, Q.; Ferrand, Y.; Chandramouli, N.; Kauffmann, B.; Aube, C.; Dubreuil, D.; Huc, I. Identification of a foldaxane kinetic byproduct during guest-induced single to double helix conversion. J. Am. Chem. Soc. 2012, 134, 15656–15659. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, X.; Kauffmann, B.; Rosu, F.; Ferrand, Y.; Huc, I. Translation of rod-like template sequences into homochiral assemblies of stacked helical oligomers. Nat. Nanotechnol. 2017, 12, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.L.; Shao, X.B.; Chen, G.J.; Zhou, Y.X.; Jiang, X.K.; Li, Z.T. Hydrogen bonded oligohydrazide foldamers and their recognition for saccharides. J. Am. Chem. Soc. 2004, 126, 12386–12394. [Google Scholar] [CrossRef]
- Inouye, M.; Waki, M.; Abe, H. Saccharide-Dependent Induction of Chiral Helicity in Achiral Synthetic Hydrogen-Bonding Oligomers. J. Am. Chem. Soc. 2004, 126, 2022–2027. [Google Scholar] [CrossRef]
- Abe, H.; Machiguchi, H.; Matsumoto, S.; Inouye, M. Saccharide recognition-induced transformation of pyridine-pyridone alternate oligomers from self-dimer to helical complex. J. Org. Chem. 2008, 73, 4650–4661. [Google Scholar] [CrossRef]
- Liu, J.; Sun, C.; Ma, W.; Lu, Y.J.; Yu, L.; Zhang, K.; Zeng, H. A conformationally switchable fluorescent oligophenol foldamer for selective sensing of copper(ii) ions. RSC Adv. 2014, 4, 54469–54473. [Google Scholar] [CrossRef]
- Hein, R.; Borissov, A.; Smith, M.D.; Beer, P.D.; Davis, J.J. A halogen-bonding foldamer molecular film for selective reagentless anion sensing in water. Chem. Commun. 2019, 55, 4849–4852. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.B.; Ma, X.Q.; Fan, Y.Q.; Jiang, X.M.; Dong, H.Q.; Yang, Q.Y.; Zhang, Y.F.; Yao, H.; Lin, Q.; Zhang, Y.M. Aggregation-induced emission supramolecular organic framework (AIE SOF) gels constructed from tri-pillar(5)arene-based foldamer for ultrasensitive detection and separation of multi-analytes. Soft Matter 2019, 15, 6753–6758. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.; Nanjan, P.; Porel, M. Sequence-defined oligomer as a modular platform for selective sub-picomolar detection and removal of Hg2+. Polym. Chem. 2021, 12, 5201–5208. [Google Scholar] [CrossRef]
- Xiong, J.B.; Feng, H.T.; Wang, J.H.; Zhang, C.; Li, B.; Zheng, Y.S. Tetraphenylethylene Foldamers with Double Hairpin-Turn Linkers, TNT-Binding Mode and Detection of Highly Diluted TNT Vapor. Chem.—Eur. J. 2018, 24, 2004–2012. [Google Scholar] [CrossRef]
- Olajos, G.; Bartus, É.; Schuster, I.; Lautner, G.; Gyurcsányi, R.E.; Szögi, T.; Fülöp, L.; Martinek, T.A. Multivalent foldamer-based affinity assay for selective recognition of Aβ oligomers. Anal. Chim. Acta 2017, 960, 131–137. [Google Scholar] [CrossRef] [Green Version]
- De los Santos, Z.A.; Yusin, G.; Wolf, C. Enantioselective sensing of carboxylic acids with a bis(urea)oligo(phenylene)ethynylene foldamer. Tetrahedron 2019, 75, 1504–1509. [Google Scholar] [CrossRef]
- Rhaman, M.M.; Hasan, M.H.; Alamgir, A.; Xu, L.; Powell, D.R.; Wong, B.M.; Tandon, R.; Hossain, M.A. Highly selective and sensitive macrocycle-based dinuclear foldamer for fluorometric and colorimetric sensing of citrate in water. Sci. Rep. 2018, 8, 286. [Google Scholar] [CrossRef] [Green Version]
- Gunasekara, R.W.; Zhao, Y. Conformationally switchable water-soluble fluorescent bischolate foldamers as membrane-curvature sensors. Langmuir 2015, 31, 3919–3925. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhu, H.; Lin, Y.; Chen, Y.; Fu, N. A Ca2+ controlled thioether linked bichromophoric squaraine foldamer for “turn on” fluorescent sensing of oxalate. Sens. Actuators B Chem. 2015, 206, 624–629. [Google Scholar] [CrossRef]
- Mateus, P.; Jacquet, A.; Méndez-Ardoy, A.; Boulloy, A.; Kauffmann, B.; Pecastaings, G.; Buffeteau, T.; Ferrand, Y.; Bassani, D.M.; Huc, I. Sensing a binding event through charge transport variations using an aromatic oligoamide capsule. Chem. Sci. 2021, 12, 3743–3750. [Google Scholar] [CrossRef] [PubMed]















| Foldamer | Sensing | Analyte | Characteristics | Ref. |
|---|---|---|---|---|
| Methionine-cholate hexamer | Fluorescence | Hg2+ | Organic solvents Linear range 0–0.24 μM Dansyl fluorescent dye | [182] |
| Hexameric oligophenol | Fluorescence | Cu2+ | THF with 1% DMSO solution 90% fluorescence quenching λex = 351 nm | [210] |
| Tetratriazole | Impedance-derived capacitance spectroscopy | ReO4− I− SCN− | LOD: 28 µM (XB), 80 µM (HB) 14 µM (XB), 47 µM (HB) 42 µM (XB), 113 µM (HB) H2O with 100 mM NaCl solution | [211] |
| Tri-pillar[5]arene (FSOF) FSOF-Cr FSOF-Fe | Fluorescence | Ions | LOD: 1.18 nM Fe3 1.86 nM Cr3+ 0.94 nM Hg2+ 1.78 nM H2PO4− 2.12 nM CN− | [212] |
| Dithiocarbamate | Fluorescence | Hg2+ | LOD = 3 10−13 M λex = 278 nm, λem = 326 nm and 339 nm. Selectivity in presence of other ions | [213] |
| Tetraphenylethylene with hairpin linkers | Fluorescence | 2,4,6-trinitrotoluene (TNT) | LOD = 0.88 fg/L of air Fluorescence quenching with increasing of TNT | [214] |
| β-peptide | Immunoassay | Aβ-oligomers | LOD = 5 pM Linear range: 10–500 pM | [215] |
| Bis(urea)oligo(phenylene)ethylene | Circular dichroism | Carboxylic acids | λmax = 370 nm Linear correlation for CD amplitude: −100–100 %ee %ee of tartaric acid: 0.2–6.4 %error | [216] |
| Dinuclear macrocycle-based copper complex | Fluorescence, colorimetry | Citrate | LOD = 0.45 ± 0.02 µM in water (pH = 7) Linear range: 1.25–8.60 µM λex = 470 nm, λem = 536 nm | [217] |
| Bis-cholate | Fluorescence | Membrane curvature | PBS buffer (pH = 7.4) λexc = 470 nm λem = 521–550 nm The binding affinity defined as Kp (103 M−1), max. = 77 ± 10 4-fold when liposome size change | [218] |
| Thioether linked biochromatic squarine | Fluorescence | Oxalate | LOD = 5.2 nM Before binding: λabs = 627–622 nm, λem = 657–687 nm After binding: Decrease λabs = 635 nm, shifted band λabs = 565 nm Decrease λem = 652 nm | [219] |
| Aromatic oligoamide | Conductivity | L-tartaric acid tetrafluorosuccinic acid, 2,2-difluorosuccinic acid | 80-fold variation of its conductance upon binding was detected by AFM | [220] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuffrida, S.G.; Forysiak, W.; Cwynar, P.; Szweda, R. Shaping Macromolecules for Sensing Applications—From Polymer Hydrogels to Foldamers. Polymers 2022, 14, 580. https://doi.org/10.3390/polym14030580
Giuffrida SG, Forysiak W, Cwynar P, Szweda R. Shaping Macromolecules for Sensing Applications—From Polymer Hydrogels to Foldamers. Polymers. 2022; 14(3):580. https://doi.org/10.3390/polym14030580
Chicago/Turabian StyleGiuffrida, Simone Giuseppe, Weronika Forysiak, Pawel Cwynar, and Roza Szweda. 2022. "Shaping Macromolecules for Sensing Applications—From Polymer Hydrogels to Foldamers" Polymers 14, no. 3: 580. https://doi.org/10.3390/polym14030580
APA StyleGiuffrida, S. G., Forysiak, W., Cwynar, P., & Szweda, R. (2022). Shaping Macromolecules for Sensing Applications—From Polymer Hydrogels to Foldamers. Polymers, 14(3), 580. https://doi.org/10.3390/polym14030580