Zn-containing Adhesives Facilitate Collagen Protection and Remineralization at the Resin-Dentin Interface: A Narrative Review
Abstract
:1. Introduction
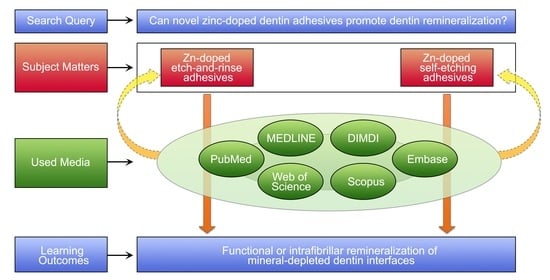
2. Methods: Literature Search
3. Results
3.1. Zn-Doped Adhesives Effects on the Dentin Bonded Interface
3.2. Matrix Metalloproteinases and Collagen Degradation. The Role of Zn as MMPs Inhibitor
3.3. Measuring ICTP as the Most Reliable Indicator of MMP-Driven Collagenolysis in Dentin
3.4. Dentin Remineralization. Zn-Substituted Apatite Compounds
4. Discussion
4.1. Zn-Doped Adhesives Effects on the Dentin Bonded Interface
4.2. Matrix Metalloproteinases and Collagen Degradation. The Role of Zn as MMPs Inhibitor
4.3. Measuring ICTP as the Most Reliable Indicator of MMP-Driven Collagenolysis in Dentin
4.4. Dentin Remineralization. Zn-Substituted Apatite Compounds
4.5. Limitations of the Present Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACP | Amorphous calcium phosphate |
| ADAM | A disintegrin and metalloproteinase |
| ADAMT | A disintegrin and metalloproteinase with thrombospondin motifs |
| CLSM | Confocal laser scanning microscopy |
| ECM | Extracellular matrix |
| EDX | Energy-dispersive X-ray spectroscopy |
| FESEM | Field Emission Scanning Electron Microscopy |
| HAP | Hydroxyapatite |
| HEMA | Hydroxyethylmethacrylate |
| ICTP | Carboxy terminal telopeptide of type I collagen |
| MDP | Methacryloyloxydecyl dihydrogen phosphate |
| MMPs | Matrix metalloproteinases |
| μXRD2 | Micro-X-ray diffractometry |
| PA | Phosphoric acid |
| TEM/SAED | Transmission Electron Microscopy/selected area diffraction |
| TIMP | Tissue inhibitor of metalloproteinases |
| TMR | Transversal microradiography |
References
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the Art Etch-and-Rinse Adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Osorio, R.; Aguilera, F.S.; Otero, P.R.; Romero, M.; Osorio, E.; García-Godoy, F.; Toledano, M. Primary Dentin Etching Time, Bond Strength and Ultra-Structure Characterization of Dentin Surfaces. J. Dent. 2010, 38, 222–231. [Google Scholar] [CrossRef]
- Hebling, J.; Pashley, D.H.; Tjäderhane, L.; Tay, F.R. Chlorhexidine Arrests Subclinical Degradation of Dentin Hybrid Layers In Vivo. J. Dent. Res. 2005, 84, 741–746. [Google Scholar] [CrossRef]
- Breschi, L.; Mazzoni, A.; Nato, F.; Carrilho, M.; Visintini, E.; Tjäderhane, L.; Ruggeri, A.; Tay, F.R.; Dorigo, E.D.S.; Pashley, D.H. Chlorhexidine Stabilizes the Adhesive Interface: A 2-Year in Vitro Study. Dent. Mater. 2010, 26, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Chaussain-Miller, C.; Fioretti, F.; Goldberg, M.; Menashi, S. The Role of Matrix Metalloproteinases (MMPs) in Human Caries. J. Dent. Res. 2006, 85, 22–32. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Park, J.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F.; et al. Adhesive/Dentin Interface: The Weak Link in the Composite Restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef] [Green Version]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Cadenaro, M.; Tjäderhane, L.; Pashley, D.H.; Tay, F.R.; Mazzoni, A. Dentin Bonding Systems: From Dentin Collagen Structure to Bond Preservation and Clinical Applications. Dent. Mater. 2018, 34, 78–96. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.F.; Marques, M.R.; Da Rosa, W.L.D.O.; Tarquinio, S.B.C.; Rosalen, P.L.; Barros, S.P. Biological Response to Self-Etch Adhesive after Partial Caries Removal in Rats. Clin. Oral Investig. 2018, 22, 2161–2173. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, R.; Osorio, E.; García-Godoy, F.; Toledano-Osorio, M.; Aguilera, F.S. Advanced Zinc-Doped Adhesives for High Performance at the Resin-Carious Dentin Interface. J. Mech. Behav. Biomed. Mater. 2016, 62, 247–267. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-S.; Kim, J.; Choi, K.-K.; Kim, S.-Y. The Influence of Chlorhexidine on the Remineralization of Demineralized Dentine. J. Dent. 2011, 39, 855–862. [Google Scholar] [CrossRef]
- Kim, Y.K.; Mai, S.; Mazzoni, A.; Liu, Y.; Tezvergil-Mutluay, A.; Takahashi, K.; Zhang, K.; Pashley, D.H.; Tay, F.R. Biomimetic Remineralization as a Progressive Dehydration Mechanism of Collagen Matrices—Implications in the Aging of Resin-Dentin Bonds. Acta Biomater. 2010, 6, 3729–3739. [Google Scholar] [CrossRef] [Green Version]
- Burwell, A.K.; Thula-Mata, T.; Gower, L.B.; Habelitz, S.; Habeliz, S.; Kurylo, M.; Ho, S.P.; Chien, Y.-C.; Cheng, J.; Cheng, N.F.; et al. Functional Remineralization of Dentin Lesions Using Polymer-Induced Liquid-Precursor Process. PLoS ONE 2012, 7, e38852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Meerbeek, B.; Dhem, A.; Goret-Nicaise, M.; Braem, M.; Lambrechts, P.; Vanherle, G. Comparative SEM and TEM Examination of the Ultrastructure of the Resin-Dentin Interdiffusion Zone. J. Dent. Res. 1993, 72, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Sauro, S.; Cabello, I.; Watson, T.; Osorio, R. A Zn-Doped Etch-and-Rinse Adhesive May Improve the Mechanical Properties and the Integrity at the Bonded-Dentin Interface. Dent. Mater. 2013, 29, e142–e152. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y. Cross-Linked Demineralized Dentin Maintains Its Mechanical Stability When Challenged by Bacterial Collagenase. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, B.H.; Feagin, F.F.; McCurdy, S.P.; Sheetz, J.H.; Speirs, R. Effects of Phosphoprotein Moieties on the Remineralization of Human Root Caries. Caries Res. 1991, 25, 166–173. [Google Scholar]
- McCurdy, S.P.; Clarkson, B.H.; Speirs, R.L.; Feagin, F.F. Phosphoprotein Extraction from the Dentine/Cementum Complex of Human Tooth Roots. Arch. Oral Biol. 1990, 35, 347–357. [Google Scholar] [CrossRef]
- Kawasaki, K.; Ruben, J.; Stokroos, I.; Takagi, O.; Arends, J. The Remineralization of EDTA-Treated Human Dentine. Caries Res. 1999, 33, 275–280. [Google Scholar] [CrossRef]
- Sauro, S.; Osorio, R.; Watson, T.F.; Toledano, M. Influence of Phosphoproteins’ Biomimetic Analogs on Remineralization of Mineral-Depleted Resin-Dentin Interfaces Created with Ion-Releasing Resin-Based Systems. Dent. Mater. 2015, 31, 759–777. [Google Scholar] [CrossRef]
- He, G.; George, A. Dentin Matrix Protein 1 Immobilized on Type I Collagen Fibrils Facilitates Apatite Deposition In Vitro. J. Biol. Chem. 2004, 279, 11649–11656. [Google Scholar] [CrossRef] [Green Version]
- Cölfen, H. Biomineralization: A Crystal-Clear View. Nat. Mater. 2010, 9, 960–961. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.H.H.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A.J.; de With, G.; Sommerdijk, N.A.J.M. The Role of Collagen in Bone Apatite Formation in the Presence of Hydroxyapatite Nucleation Inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daood, U.; Iqbal, K.; Nitisusanta, L.I.; Fawzy, A.S. Effect of Chitosan/Riboflavin Modification on Resin/Dentin Interface: Spectroscopic and Microscopic Investigations. J. Biomed. Mater. Res. A 2013, 101, 1846–1856. [Google Scholar] [CrossRef]
- Hao, J.; Ramachandran, A.; George, A. Temporal and Spatial Localization of the Dentin Matrix Proteins during Dentin Biomineralization. J. Histochem. Cytochem. 2009, 57, 227–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linde, A.; Lussi, A.; Crenshaw, M.A. Mineral Induction by Immobilized Polyanionic Proteins. Calcif. Tissue Int. 1989, 44, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Neoh, K.G.; Lin, C.C.; Kishen, A. Biomimetic Deposition of Calcium Phosphate Minerals on the Surface of Partially Demineralized Dentine Modified with Phosphorylated Chitosan. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Scheven, B.A.; Takahashi, Y.; Ferracane, J.L.; Shelton, R.M.; Cooper, P.R. Dentine as a Bioactive Extracellular Matrix. Arch. Oral Biol. 2012, 57, 109–121. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Habelitz, S.; Pugach, M.; Soares, P.C.; Marshall, S.J.; Marshall, G.W. Evaluation of Surface Structural and Mechanical Changes Following Remineralization of Dentin. Scanning 2010, 32, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Bertassoni, L.E.; Habelitz, S.; Kinney, J.H.; Marshall, S.J.; Marshall, G.W. Biomechanical Perspective on the Remineralization of Dentin. Caries Res. 2009, 43, 70–77. [Google Scholar] [CrossRef]
- Toledano, M.; Cabello, I.; Vílchez, M.A.C.; Fernández, M.A.; Osorio, R. Surface Microanalysis and Chemical Imaging of Early Dentin Remineralization. Microsc. Microanal. 2014, 20, 245–256. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, R.; Osorio, E.; Cabello, I.; Toledano-Osorio, M.; Aguilera, F.S. A Zinc Chloride-Doped Adhesive Facilitates Sealing at the Dentin Interface: A Confocal Laser Microscopy Study. J. Mech. Behav. Biomed. Mater. 2017, 74, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcellos, D.C.; Fonseca, B.M.; Pucci, C.R.; das Neves Cavalcanti, B.; Persici, E.D.S.; de Paiva Gonçalves, S.E. Zn-Doped Etch-and-Rinse Model Dentin Adhesives: Dentin Bond Integrity, Biocompatibility, and Properties. Dent. Mater. 2016, 32, 940–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio, R.; Osorio, E.; Medina-Castillo, A.L.; Toledano, M. Polymer Nanocarriers for Dentin Adhesion. J. Dent. Res. 2014, 93, 1258–1263. [Google Scholar] [CrossRef] [Green Version]
- Toledano, M.; Gutierrez-Pérez, J.L.; Gutierrez-Corrales, A.; Serrera-Figallo, M.A.; Toledano-Osorio, M.; Rosales-Leal, J.I.; Aguilar, M.; Osorio, R.; Torres-Lagares, D. Novel Non-Resorbable Polymeric-Nanostructured Scaffolds for Guided Bone Regeneration. Clin. Oral Investig. 2019, 24, 2037–2049. [Google Scholar] [CrossRef]
- Osorio, R.; Yamauti, M.; Osorio, E.; Román, J.S.; Toledano, M. Zinc-Doped Dentin Adhesive for Collagen Protection at the Hybrid Layer. Eur. J. Oral Sci. 2011, 119, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A Review of the Biological Response to Ionic Dissolution Products from Bioactive Glasses and Glass-Ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Lynch, R.J.M.; Churchley, D.; Butler, A.; Kearns, S.; Thomas, G.V.; Badrock, T.C.; Cooper, L.; Higham, S.M. Effects of Zinc and Fluoride on the Remineralisation of Artificial Carious Lesions under Simulated Plaque Fluid Conditions. Caries Res. 2011, 45, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Takatsuka, T.; Tanaka, K.; Iijima, Y. Inhibition of Dentine Demineralization by Zinc Oxide: In Vitro and in Situ Studies. Dent. Mater. 2005, 21, 1170–1177. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions Able to Reproduce In Vivo Surface-Structure Changes in Bioactive Glass-Ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Gutiérrez, M.F.; Bermudez, J.; Dávila-Sánchez, A.; Alegría-Acevedo, L.F.; Méndez-Bauer, L.; Hernández, M.; Astorga, J.; Reis, A.; Loguercio, A.D.; Farago, P.V.; et al. Zinc Oxide and Copper Nanoparticles Addition in Universal Adhesive Systems Improve Interface Stability on Caries-Affected Dentin. J. Mech. Behav. Biomed. Mater. 2019, 100, 103366. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Toledano-Osorio, M.; Bueno, J.; Figuero, E.; Toledano, M.; Medina-Castillo, A.L.; Osorio, R.; Herrera, D.; Sanz, M. Antibacterial Effects of Polymeric PolymP-n Active Nanoparticles. An In Vitro Biofilm Study. Dent. Mater. 2019, 35, 156–168. [Google Scholar] [CrossRef]
- Toledano, M.; Toledano-Osorio, M.; Osorio, R.; Carrasco-Carmona, Á.; Gutiérrez-Pérez, J.-L.; Gutiérrez-Corrales, A.; Serrera-Figallo, M.-A.; Lynch, C.D.; Torres-Lagares, D. Doxycycline and Zinc Loaded Silica-Nanofibrous Polymers as Biomaterials for Bone Regeneration. Polymers 2020, 12, 1201. [Google Scholar] [CrossRef]
- Hadley, K.B.; Newman, S.M.; Hunt, J.R. Dietary Zinc Reduces Osteoclast Resorption Activities and Increases Markers of Osteoblast Differentiation, Matrix Maturation, and Mineralization in the Long Bones of Growing Rats. J. Nutr. Biochem. 2010, 21, 297–303. [Google Scholar] [CrossRef]
- Toledano, M.; Aguilera, F.S.; Osorio, E.; Cabello, I.; Toledano-Osorio, M.; Osorio, R. Functional and Molecular Structural Analysis of Dentine Interfaces Promoted by a Zn-Doped Self-Etching Adhesive and an in Vitro Load Cycling Model. J. Mech. Behav. Biomed. Mater. 2015, 50, 131–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, I.M.; Leitune, V.C.B.; Visioli, F.; Samuel, S.M.W.; Collares, F.M. Influence of Zinc Oxide Quantum Dots in the Antibacterial Activity and Cytotoxicity of an Experimental Adhesive Resin. J. Dent. 2018, 73, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.M.P.; Oliveira, C.A.R.; Macedo, J.P.C.; França, F.M.G.; Basting, R.T.; Turssi, C.P.; Silva, T.M.; Gonçalves, S.E.P.; Amaral, F.L.B. Effect of Zinc Chloride Added to Self-Etching Primer on Bond Strength to Caries-Affected Dentin and Chemical-Physical-Mechanical Properties of Adhesives. Int. J. Adhes. Adhes. 2019, 95, 102412. [Google Scholar] [CrossRef]
- Osorio, R.; Cabello, I.; Toledano, M. Bioactivity of Zinc-Doped Dental Adhesives. J. Dent. 2014, 42, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Mannocci, F.; Toledano, M.; Osorio, R.; Pashley, D.H.; Watson, T.F. EDTA or H3PO4/NaOCl Dentine Treatments May Increase Hybrid Layers’ Resistance to Degradation: A Microtensile Bond Strength and Confocal-Micropermeability Study. J. Dent. 2009, 37, 279–288. [Google Scholar] [CrossRef]
- Osorio, R.; Yamauti, M.; Osorio, E.; Ruiz-Requena, M.E.; Pashley, D.H.; Tay, F.; Toledano, M. Zinc Reduces Collagen Degradation in Demineralized Human Dentin Explants. J. Dent. 2011, 39, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Osorio, R.; Yamauti, M.; Osorio, E.; Ruiz-Requena, M.E.; Pashley, D.; Tay, F.; Toledano, M. Effect of Dentin Etching and Chlorhexidine Application on Metalloproteinase-Mediated Collagen Degradation. Eur. J. Oral Sci. 2011, 119, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Osorio, R.; Osorio, E.; Cabello, I.; Toledano, M. Zinc Induces Apatite and Scholzite Formation during Dentin Remineralization. Caries Res. 2014, 48, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Yamauti, M.; Ruiz-Requena, M.E.; Osorio, R. A ZnO-Doped Adhesive Reduced Collagen Degradation Favouring Dentine Remineralization. J. Dent. 2012, 40, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Yamasaki, S.; Nishida, K.; Murakami, M.; Hirano, T. Zinc Homeostasis and Signaling in Health and Diseases: Zinc Signaling. J. Biol. Inorg. Chem. 2011, 16, 1123–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hume, W.R. An Analysis of the Release and the Diffusion through Dentin of Eugenol from Zinc Oxide-Eugenol Mixtures. J. Dent. Res. 1984, 63, 881–884. [Google Scholar] [CrossRef]
- Seseogullari-Dirihan, R.; Mutluay, M.M.; Pashley, D.H.; Tezvergil-Mutluay, A. Is the Inactivation of Dentin Proteases by Crosslinkers Reversible? Dent. Mater. 2017, 33, e62–e68. [Google Scholar] [CrossRef]
- Braga, R.R.; Fronza, B.M. The Use of Bioactive Particles and Biomimetic Analogues for Increasing the Longevity of Resin-Dentin Interfaces: A Literature Review. Dent. Mater. J. 2020, 39, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Toledano, M.; Aguilera, F.S.; Osorio, E.; Cabello, I.; Toledano-Osorio, M.; Osorio, R. Bond Strength and Bioactivity of Zn-Doped Dental Adhesives Promoted by Load Cycling. Microsc. Microanal. 2015, 21, 214–230. [Google Scholar] [CrossRef]
- Wang, L.; Chen, F.; Yang, F.; Hoshika, S.; Yamauti, M.; Liu, Y.; Sano, H. Bioactive Two-Step Approach: Promising Bonding Strategy for a One-Step Self-Etch Universal Adhesive. J. Adhes. Dent. 2019, 21, 413–421. [Google Scholar]
- Gutiérrez, M.F.; Alegría-Acevedo, L.F.; Méndez-Bauer, L.; Bermudez, J.; Dávila-Sánchez, A.; Buvinic, S.; Hernández-Moya, N.; Reis, A.; Loguercio, A.D.; Farago, P.V.; et al. Biological, Mechanical and Adhesive Properties of Universal Adhesives Containing Zinc and Copper Nanoparticles. J. Dent. 2019, 82, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Pomacóndor-Hernández, C.; Osorio, R.; Aguilera, F.S.; Cabello, I.; De Goes, M.; Toledano, M. Effect of Zinc-Doping in Physicochemical Properties of Dental Adhesives. Am. J. Dent. 2015, 28, 292–296. [Google Scholar]
- Oh, J.-H.; Kim, H.-J.; Kim, T.-I.; Baek, J.-H.; Ryoo, H.-M.; Woo, K.M. The Effects of the Modulation of the Fibronectin-Binding Capacity of Fibrin by Thrombin on Osteoblast Differentiation. Biomaterials 2012, 33, 4089–4099. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Toledano-Osorio, M.; Osorio, E.; Aguilera, F.S.; Padilla-Mondéjar, S.; Toledano, M. Zinc and Silica Are Active Components to Efficiently Treat in Vitro Simulated Eroded Dentin. Clin. Oral Investig. 2018, 22, 2859–2870. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Cabello, I.; Osorio, E.; Aguilera, F.S.; Medina-Castillo, A.L.; Toledano-Osorio, M.; Osorio, R. Zn-Containing Polymer Nanogels Promote Cervical Dentin Remineralization. Clin. Oral Investig. 2019, 23, 1197–1208. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; Osorio, E.; Aguilera, F.S.; Medina-Castillo, A.L.; Toledano, M.; Osorio, R. Improved Reactive Nanoparticles to Treat Dentin Hypersensitivity. Acta Biomater. 2018, 72, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Nyvad, B.; Crielaard, W.; Mira, A.; Takahashi, N.; Beighton, D. Dental Caries from a Molecular Microbiological Perspective. Caries Res. 2013, 47, 89–102. [Google Scholar] [CrossRef]
- Brown, I.D. The Chemical Bond in Inorganic Chemistry: The Bond Valence Model; Oxford University Press: Oxford, UK, 2006; ISBN 978-0-19-929881-5. [Google Scholar]
- Almeida, G.; da Silva, E.; Guimarães, J.G.; Silva, R.; Santos, G.; Poskus, L. ZnCl 2 Incorporated into Experimental Adhesives: Selected Physicochemical Properties and Resin-Dentin Bonding Stability. BioMed Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Toledano, M.; Aguilera, F.S.; Osorio, E.; Cabello, I.; Toledano-Osorio, M.; Osorio, R. Self-Etching Zinc-Doped Adhesives Improve the Potential of Caries-Affected Dentin to Be Functionally Remineralized. Biointerphases 2015, 10, 031002. [Google Scholar] [CrossRef]
- Feitosa, V.P.; Pomacóndor-Hernández, C.; Ogliari, F.A.; Leal, F.; Correr, A.B.; Sauro, S. Chemical Interaction of 10-MDP (Methacryloyloxi-Decyl-Dihydrogen-Phosphate) in Zinc-Doped Self-Etch Adhesives. J. Dent. 2014, 42, 359–365. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Pietrzykowska, A.; Zalewska, A.; Knaś, M.; Daniszewska, I. The Significance of Matrix Metalloproteinases in Oral Diseases. Adv. Clin. Exp. Med. 2016, 25, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Betancourt, D.; Baldion, P.; Castellanos, J. Resin-Dentin Bonding Interface: Mechanisms of Degradation and Strategies for Stabilization of the Hybrid Layer. Int. J. Biomater. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.S.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.; Carvalho, R.M.; Tay, F.R.; et al. Optimizing Dentin Bond Durability: Strategies to Prevent Hydrolytic Degradation of the Hybrid Layer. Dent. Mater. 2013, 29, 999–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledano, M.; Nieto-Aguilar, R.; Osorio, R.; Campos, A.; Osorio, E.; Tay, F.R.; Alaminos, M. Differential Expression of Matrix Metalloproteinase-2 in Human Coronal and Radicular Sound and Carious Dentine. J. Dent. 2010, 38, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Geurink, P.P.; Overkleeft, H.S.; K Kauffman, H.; Bischoff, R. Functional Proteomics on Zinc-Dependent Metalloproteinases Using Inhibitor Probes. ChemMedChem 2009, 4, 164–170. [Google Scholar] [CrossRef] [PubMed]
- McCall, K.A.; Huang, C.; Fierke, C.A. Function and Mechanism of Zinc Metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.; Jung, H.-S.; Kim, H.-J.; Jang, J.-H.; Kim, D.-S.; Choi, K.-K.; Kim, S.-Y. Effect of Zinc on the Collagen Degradation in Acid-Etched Dentin. J. Dent. Sci. 2018, 13, 97–102. [Google Scholar] [CrossRef]
- Toledano, M.; Yamauti, M.; Osorio, E.; Osorio, R. Zinc-Inhibited MMP-Mediated Collagen Degradation after Different Dentine Demineralization Procedures. Caries Res. 2012, 46, 201–207. [Google Scholar] [CrossRef]
- Larsen, K.S.; Auld, D.S. Characterization of an Inhibitory Metal Binding Site in Carboxypeptidase A. Biochemistry 1991, 30, 2613–2618. [Google Scholar] [CrossRef]
- Sakharov, D.V.; Lim, C. Zn Protein Simulations Including Charge Transfer and Local Polarization Effects. J. Am. Chem. Soc. 2005, 127, 4921–4929. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, K.; Olsson, H.; Mörgelin, M.; Heinegård, D. Cartilage Oligomeric Matrix Protein Shows High Affinity Zinc-Dependent Interaction with Triple Helical Collagen. J. Biol. Chem. 1998, 273, 20397–20403. [Google Scholar] [CrossRef] [Green Version]
- Dzamba, B.J.; Wu, H.; Jaenisch, R.; Peters, D.M. Fibronectin Binding Site in Type I Collagen Regulates Fibronectin Fibril Formation. J. Cell Biol. 1993, 121, 1165–1172. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.-P.; Zou, C.; Yuan, X.; Luo, W.; Wen, Y.; Chen, Y. N-Terminal Modification Increases the Stability of the Recombinant Human Endostatin In Vitro. Biotechnol. Appl. Biochem. 2009, 54, 113–120. [Google Scholar] [CrossRef]
- Lenglet, S.; Mach, F.; Montecucco, F. Role of Matrix Metalloproteinase-8 in Atherosclerosis. Mediat. Inflamm. 2013, 2013, 659282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brew, K.; Nagase, H. The Tissue Inhibitors of Metalloproteinases (TIMPs): An Ancient Family with Structural and Functional Diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skarja, G.A.; Brown, A.L.; Ho, R.K.; May, M.H.; Sefton, M.V. The Effect of a Hydroxamic Acid-Containing Polymer on Active Matrix Metalloproteinases. Biomaterials 2009, 30, 1890–1897. [Google Scholar] [CrossRef]
- De Munck, J.; Van Meerbeek, B.; Yoshida, Y.; Inoue, S.; Vargas, M.; Suzuki, K.; Lambrechts, P.; Vanherle, G. Four-Year Water Degradation of Total-Etch Adhesives Bonded to Dentin. J. Dent. Res. 2003, 82, 136–140. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, R.; Albaladejo, A.; Aguilera, F.S.; Osorio, E. Differential Effect of in Vitro Degradation on Resin-Dentin Bonds Produced by Self-Etch versus Total-Etch Adhesives. J. Biomed. Mater. Res. A 2006, 77, 128–135. [Google Scholar] [CrossRef]
- Garnero, P.; Ferreras, M.; Karsdal, M.A.; Nicamhlaoibh, R.; Risteli, J.; Borel, O.; Qvist, P.; Delmas, P.D.; Foged, N.T.; Delaissé, J.M. The Type I Collagen Fragments ICTP and CTX Reveal Distinct Enzymatic Pathways of Bone Collagen Degradation. J. Bone Miner. Res. 2003, 18, 859–867. [Google Scholar] [CrossRef]
- Okabe, R.; Nakatsuka, K.; Inaba, M.; Miki, T.; Naka, H.; Masaki, H.; Moriguchi, A.; Nishizawa, Y. Clinical Evaluation of the Elecsys Beta-CrossLaps Serum Assay, a New Assay for Degradation Products of Type I Collagen C-Tlopeptides. Clin. Chem. 2001, 47, 1410–1414. [Google Scholar] [CrossRef]
- Piecha, D.; Weik, J.; Kheil, H.; Becher, G.; Timmermann, A.; Jaworski, A.; Burger, M.; Hofmann, M.W. Novel Selective MMP-13 Inhibitors Reduce Collagen Degradation in Bovine Articular and Human Osteoarthritis Cartilage Explants. Inflamm. Res. 2010, 59, 379–389. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, R. New Advanced Materials for High Performance at the Resin-Dentine Interface. Front. Oral Biol. 2015, 17, 39–48. [Google Scholar]
- Oudadesse, H.; Dietrich, E.; Gal, Y.L.; Pellen, P.; Bureau, B.; Mostafa, A.A.; Cathelineau, G. Apatite Forming Ability and Cytocompatibility of Pure and Zn-Doped Bioactive Glasses. Biomed. Mater. 2011, 6, 035006. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Ling, J.; Legeros, J.; LeGeros, R. Calcium Phosphate-Based Solutions Promote Dentin Tubule Occlusions Less Susceptible to Acid Dissolution. Am. J. Dent. 2011, 24, 169–175. [Google Scholar]
- Makowski, G.S.; Ramsby, M.L. Differential Effect of Calcium Phosphate and Calcium Pyrophosphate on Binding of Matrix Metalloproteinases to Fibrin: Comparison to a Fibrin-Binding Protease from Inflammatory Joint Fluids. Clin. Exp. Immun. 2004, 136, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Kremer, E.A.; Chen, Y.; Suzuki, K.; Nagase, H.; Gorski, J.P. Hydroxyapatite Induces Autolytic Degradation and Inactivation of Matrix Metalloproteinase-1 and -3. J. Bone Min. Res. 1998, 13, 1890–1902. [Google Scholar] [CrossRef]
- Tallant, C.; Marrero, A.; Gomis-Rüth, F.X. Matrix Metalloproteinases: Fold and Function of Their Catalytic Domains. Biochim. Biophys. Acta 2010, 1803, 20–28. [Google Scholar] [CrossRef]
- Boukpessi, T.; Menashi, S.; Camoin, L.; Tencate, J.M.; Goldberg, M.; Chaussain-Miller, C. The Effect of Stromelysin-1 (MMP-3) on Non-Collagenous Extracellular Matrix Proteins of Demineralized Dentin and the Adhesive Properties of Restorative Resins. Biomaterials 2008, 29, 4367–4373. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Larjava, H.; Sorsa, T.; Uitto, V.J.; Larmas, M.; Salo, T. The Activation and Function of Host Matrix Metalloproteinases in Dentin Matrix Breakdown in Caries Lesions. J. Dent. Res. 1998, 77, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Lamparter, S.; Slight, S.H.; Weber, K.T. Doxycycline and Tissue Repair in Rats. J. Lab. Clin. Med. 2002, 139, 295–302. [Google Scholar] [CrossRef]
- Carrilho, M.R.; Tay, F.R.; Donnelly, A.M.; Agee, K.A.; Tjäderhane, L.; Mazzoni, A.; Breschi, L.; Foulger, S.; Pashley, D.H. Host-Derived Loss of Dentin Matrix Stiffness Associated with Solubilization of Collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, N.; Debret, R.; Roméas, A.; Magloire, H.; Degrange, M.; Bleicher, F.; Sommer, P.; Seux, D. Self-Etching Increases Matrix Metalloproteinase Expression in the Dentin-Pulp Complex. J. Dent. Res. 2009, 88, 77–82. [Google Scholar] [CrossRef]
- Mazzoni, A.; Mannello, F.; Tay, F.R.; Tonti, G.A.M.; Papa, S.; Mazzotti, G.; Di Lenarda, R.; Pashley, D.H.; Breschi, L. Zymographic Analysis and Characterization of MMP-2 and -9 Forms in Human Sound Dentin. J. Dent. Res. 2007, 86, 436–440. [Google Scholar] [CrossRef]
- Dey, A.; Bomans, P.H.H.; Müller, F.A.; Will, J.; Frederik, P.M.; de With, G.; Sommerdijk, N.A.J.M. The Role of Prenucleation Clusters in Surface-Induced Calcium Phosphate Crystallization. Nat. Mater. 2010, 9, 1010–1014. [Google Scholar] [CrossRef]
- Lussi, A.; Linde, A. Mineral Induction in Vivo by Dentine Proteins. Caries Res. 1993, 27, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, M.T.; Brown, P.W. Effects of Na2HPO4 and NaH2PO4 on Hydroxyapatite Formation. J. Biomed. Mater. Res. 1993, 27, 1095–1102. [Google Scholar] [CrossRef]
- Tezvergil-Mutluay, A.; Agee, K.A.; Hoshika, T.; Carrilho, M.; Breschi, L.; Tjäderhane, L.; Nishitani, Y.; Carvalho, R.M.; Looney, S.; Tay, F.R.; et al. The Requirement of Zinc and Calcium Ions for Functional MMP Activity in Demineralized Dentin Matrices. Dent. Mater. 2010, 26, 1059–1067. [Google Scholar] [CrossRef] [Green Version]
- Son, J.-S.; Kim, S.-G.; Oh, J.-S.; Appleford, M.; Oh, S.; Ong, J.L.; Lee, K.-B. Hydroxyapatite/Polylactide Biphasic Combination Scaffold Loaded with Dexamethasone for Bone Regeneration. J. Biomed. Mater. Res. A 2011, 99, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Mayer, I.; Apfelbaum, F.; Featherstone, J.D.B. Zinc Ions in Synthetic Carbonated Hydroxyapatites. Arch. Oral Biol. 1994, 39, 87–90. [Google Scholar] [CrossRef]
- Horiuchi, S.; Asaoka, K.; Tanaka, E. Development of a Novel Cement by Conversion of Hopeite in Set Zinc Phosphate Cement into Biocompatible Apatite. Bio-Med. Mater. Eng. 2009, 19, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Rautray, T.R.; Narayanan, R.; Kim, K.-H. Ion Implantation of Titanium Based Biomaterials. Prog. Mater. Sci. 2011, 56, 1137–1177. [Google Scholar] [CrossRef] [Green Version]
- Heinz, U.; Hemmingsen, L.; Kiefer, M.; Adolph, H.-W. Structural Adaptability of Zinc Binding Sites: Different Structures in Partially, Fully, and Heavy-Metal Loaded States. Chemistry 2009, 15, 7350–7358. [Google Scholar] [CrossRef]
- Liu, Y.; Mai, S.; Li, N.; Yiu, C.K.Y.; Mao, J.; Pashley, D.H.; Tay, F.R. Differences between Top-down and Bottom-up Approaches in Mineralizing Thick, Partially Demineralized Collagen Scaffolds. Acta Biomater. 2011, 7, 1742–1751. [Google Scholar] [CrossRef] [Green Version]
- Carrilho, M.R.; Carvalho, R.M.; Sousa, E.N.; Nicolau, J.; Breschi, L.; Mazzoni, A.; Tjäderhane, L.; Tay, F.R.; Agee, K.; Pashley, D.H. Substantivity of Chlorhexidine to Human Dentin. Dent. Mater. 2010, 26, 779–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fawzy, A.; Nitisusanta, L.; Iqbal, K.; Daood, U.; Beng, L.T.; Neo, J. Characterization of Riboflavin-Modified Dentin Collagen Matrix. J. Dent. Res. 2012, 91, 1049–1054. [Google Scholar] [CrossRef]
- Henn, S.; de Carvalho, R.V.; Ogliari, F.A.; de Souza, A.P.; Line, S.R.P.; da Silva, A.F.; Demarco, F.F.; Etges, A.; Piva, E. Addition of Zinc Methacrylate in Dental Polymers: MMP-2 Inhibition and Ultimate Tensile Strength Evaluation. Clin. Oral Investig. 2012, 16, 531–536. [Google Scholar] [CrossRef]
- De Souza, A.P.; Gerlach, R.F.; Line, S.R. Inhibition of Human Gingival Gelatinases (MMP-2 and MMP-9) by Metal Salts. Dent. Mater. 2000, 16, 103–108. [Google Scholar] [CrossRef]
- Santos, M.C.L.G.; de Souza, A.P.; Gerlach, R.F.; Trevilatto, P.C.; Scarel-Caminaga, R.M.; Line, S.R.P. Inhibition of Human Pulpal Gelatinases (MMP-2 and MMP-9) by Zinc Oxide Cements. J. Oral Rehabil. 2004, 31, 660–664. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledano, M.; Toledano-Osorio, M.; Hannig, M.; Carrasco-Carmona, Á.; Osorio, M.T.; García-Godoy, F.; Cabello, I.; Osorio, R. Zn-containing Adhesives Facilitate Collagen Protection and Remineralization at the Resin-Dentin Interface: A Narrative Review. Polymers 2022, 14, 642. https://doi.org/10.3390/polym14030642
Toledano M, Toledano-Osorio M, Hannig M, Carrasco-Carmona Á, Osorio MT, García-Godoy F, Cabello I, Osorio R. Zn-containing Adhesives Facilitate Collagen Protection and Remineralization at the Resin-Dentin Interface: A Narrative Review. Polymers. 2022; 14(3):642. https://doi.org/10.3390/polym14030642
Chicago/Turabian StyleToledano, Manuel, Manuel Toledano-Osorio, Matthias Hannig, Álvaro Carrasco-Carmona, María T. Osorio, Franklin García-Godoy, Inmaculada Cabello, and Raquel Osorio. 2022. "Zn-containing Adhesives Facilitate Collagen Protection and Remineralization at the Resin-Dentin Interface: A Narrative Review" Polymers 14, no. 3: 642. https://doi.org/10.3390/polym14030642
APA StyleToledano, M., Toledano-Osorio, M., Hannig, M., Carrasco-Carmona, Á., Osorio, M. T., García-Godoy, F., Cabello, I., & Osorio, R. (2022). Zn-containing Adhesives Facilitate Collagen Protection and Remineralization at the Resin-Dentin Interface: A Narrative Review. Polymers, 14(3), 642. https://doi.org/10.3390/polym14030642