Biodiesel Synthesis from High Free-Fatty-Acid Chicken Fat using a Scrap-Tire Derived Solid Acid Catalyst and KOH
Abstract
:1. Introduction
2. Experimental Setup and Methodology
2.1. Preparation of a Sulfonated Tire Polymer Char (TPC-SO3H) Catalyst
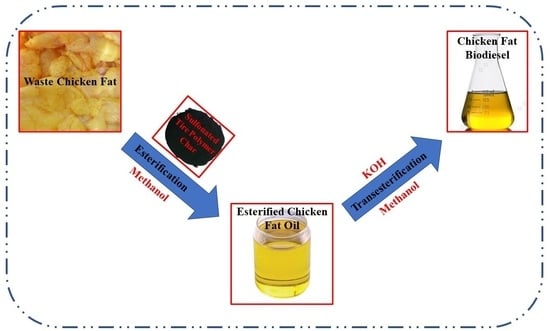
2.2. Biodiesel Synthesis
2.2.1. Chicken Fat Extraction
2.2.2. Esterification and Transesterification
2.3. Characterization Techniques
3. Results and Discussion
3.1. Catalyst Characterization
3.2. Biodiesel Production
3.2.1. Acid Catalyzed Chicken Fat Esterification (1st Stage)
3.2.2. Reusability of TPC-SO3H Catalyst
3.2.3. Catalytic Activity Comparison of the Waste-Derived Sulfonated Char Catalysts
3.2.4. Alkali Catalyzed Transesterification (2nd Stage)
3.3. Biodiesel Characterization
4. Conclusions
- In this work, we synthesized a heterogeneous carbon-based sulfonated tire polymer char TPC-SO3H solid acid catalyst from scrap tire through a two-step procedure comprising pyrolysis of scrap tire at a temperature of 500 °C followed by thermal acid treatment employing concentrated H2SO4 at 180 °C.
- The produced catalyst was then successfully utilized to synthesize biodiesel from waste chicken fat through a two-step conversion process of esterification to decrease the FFA content of chicken fat, followed by conventional alkali-catalyzed transesterification.
- The optimal esterification reaction conditions found were 70 °C and 120 min with a 15:1 methanol-to-CF molar ratio and 5 wt.% catalyst dosage.
- The synthesized catalyst performed well for the first three cycles without exhibiting any significant degradation in its catalytic activity under the ascertained optimal settings.
- The chemical and physicochemical properties of the synthesized biodiesel were found to fall under the set limits of biodiesel, suggesting the complete conversion of chicken fat into biodiesel.
- Lastly, we compared our scrap-tire derived catalyst with other previously reported waste-derived sulfonated catalysts and concluded that our synthesized catalyst produced the maximal biodiesel yield.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, J.; Agarwal, M.; Dalai, A.K. Optimization of Biodiesel Production from Mixture of Edible and Nonedible Vegetable Oils. Biocatal. Agric. Biotechnol. 2016, 8, 112–120. [Google Scholar] [CrossRef]
- Abed, K.A.; Gad, M.S.; el Morsi, A.K.; Sayed, M.M.; Elyazeed, S.A. Effect of Biodiesel Fuels on Diesel Engine Emissions. Egypt. J. Pet. 2019, 28, 183–188. [Google Scholar] [CrossRef]
- Zuleta, E.C.; Rios, L.A.; Benjumea, P.N. Oxidative Stability and Cold Flow Behavior of Palm, Sacha-Inchi, Jatropha and Castor Oil Biodiesel Blends. Fuel Processing Technol. 2012, 102, 96–101. [Google Scholar] [CrossRef]
- Islam, M.M.; Hassan, M.H.; Kalam, M.A.; Zulkifli, N.W.; Binti, M.; Habibullah, M.; Hossain, M.M. Improvement of Cold Flow Properties of Cocos Nucifera and Calophyllum Inophyllum Biodiesel Blends Using Polymethyl Acrylate Additive. J. Clean. Prod. 2016, 137, 322–329. [Google Scholar] [CrossRef]
- Veríssimo, M.I.S.; Gomes, M.T.S.R. Assessment on the Use of Biodiesel in Cold Weather: Pour Point Determination Using a Piezoelectric Quartz Crystal. Fuel 2011, 90, 2315–2320. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Schumacher, L.G.; Suppes, G.J. Impact of Cold Flow Improvers on Soybean Biodiesel Blend. Biomass Bioenergy 2004, 27, 485–491. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B. Effect of Winterization and Plant Phenolic-Additives on the Cold-Flow Properties and Oxidative Stability of Karanja Biodiesel. Fuel 2020, 262, 116631. [Google Scholar] [CrossRef]
- Dunn, R.O.; Shockley, M.W.; Bagby, M.O. Improving the Low-Temperature Properties of Alternative Diesel Fuels: Vegetable Oil-Derived Methyl Esters. J. Am. Oil Chem. Soc. 1996, 73, 1719–1728. [Google Scholar] [CrossRef]
- Magalhães, A.M.S.; Pereira, E.; Meirelles, A.J.A.; Sampaio, K.A.; Maximo, G.J. Proposing Blends for Improving the Cold Flow Properties of Ethylic Biodiesel. Fuel 2019, 253, 50–59. [Google Scholar] [CrossRef]
- Lee, I.; Johnson, L.A.; Hammond, E.G. Use of Branched-Chain Esters to Reduce the Crystallization Temperature of Biodiesel. J. Am. Oil Chem. Soc. 1995, 72, 1155–1160. [Google Scholar] [CrossRef]
- Hazrat, M.A.; Rasul, M.G.; Mofijur, M.; Khan, M.M.K.; Djavanroodi, F.; Azad, A.K.; Bhuiya, M.M.K.; Silitonga, A.S. A Mini Review on the Cold Flow Properties of Biodiesel and Its Blends. Front. Energy Res. 2020, 8, 326. [Google Scholar] [CrossRef]
- Sanjeevannavar, M.B.; Banapurmath, N.R.; Soudagar, M.E.M.; Atgur, V.; Hossain, N.; Mujtaba, M.A.; Khan, T.M.Y.; Rao, B.N.; Ismail, K.A.; Elfasakhany, A. Performance Indicators for the Optimal BTE of Biodiesels with Additives through Engine Testing by the Taguchi Approach. Chemosphere 2022, 288, 132450. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.A.; Muk Cho, H.; Masjuki, H.H.; Kalam, M.A.; Farooq, M.; Soudagar, M.E.M.; Gul, M.; Afzal, A.; Ahmed, W.; Raza, A.; et al. Effect of Primary and Secondary Alcohols as Oxygenated Additives on the Performance and Emission Characteristics of Diesel Engine. Energy Rep. 2021, 7, 1116–1124. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Ghazali, N.-N.; Abul Kalam, M.; Badruddin, I.A.; Banapurmath, N.R.; Akram, N. The Effect of Nano-Additives in Diesel-Biodiesel Fuel Blends: A Comprehensive Review on Stability, Engine Performance and Emission Characteristics. Energy Convers. Manag. 2018, 178, 146–177. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Muk Cho, H.; Masjuki, H.H.; Kalam, M.A.; Farooq, M.; Soudagar, M.E.M.; Gul, M.; Ahmed, W.; Afzal, A.; Bashir, S.; et al. Effect of Alcoholic and Nano-Particles Additives on Tribological Properties of Diesel–Palm–Sesame–Biodiesel Blends. Energy Rep. 2021, 7, 1162–1171. [Google Scholar] [CrossRef]
- Hussain, F.; Soudagar, M.E.M.; Afzal, A.; Mujtaba, M.A.; Fattah, I.M.R.; Naik, B.; Mulla, M.H.; Badruddin, I.A.; Khan, T.M.Y.; Raju, V.D.; et al. Enhancement in Combustion, Performance, and Emission Characteristics of a Diesel Engine Fueled with Ce-ZnO Nanoparticle Additive Added to Soybean Biodiesel Blends. Energies 2020, 13, 4578. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Kalam, M.A.; Masjuki, H.H.; Gul, M.; Soudagar, M.E.M.; Ong, H.C.; Ahmed, W.; Atabani, A.E.; Razzaq, L.; Yusoff, M. Comparative Study of Nanoparticles and Alcoholic Fuel Additives-Biodiesel-Diesel Blend for Performance and Emission Improvements. Fuel 2020, 279, 118434. [Google Scholar] [CrossRef]
- Soudagar, M.E.; Mujtaba, M.A.; Safaei, M.R.; Afzal, A.; Ahmed, W.; Banapurmath, N.R.; Hossain, N.; Bashir, S.; Badruddin, I.A.; Goodarzi, M.; et al. Effect of Sr@ZnO Nanoparticles and Ricinus Communis Biodiesel-Diesel Fuel Blends on Modified CRDI Diesel Engine Characteristics. Energy 2021, 215, 119094. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Tabatabaei, M.; Aghbashlo, M.; Khanali, M.; Demirbas, A. A Comprehensive Review on the Environmental Impacts of Diesel/Biodiesel Additives. Energy Convers. Manag. 2018, 174, 579–614. [Google Scholar] [CrossRef]
- Unosson, J.; Kabéle, M.; Boman, C.; Nyström, R.; Sadiktsis, I.; Westerholm, R.; Mudway, I.S.; Purdie, E.; Raftis, J.; Miller, M.R.; et al. Acute Cardiovascular Effects of Controlled Exposure to Dilute Petrodiesel and Biodiesel Exhaust in Healthy Volunteers: A Crossover Study. Part. Fibre Toxicol. 2021, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Mehus, A.A.; Reed, R.J.; Lee, V.S.T.; Littau, S.R.; Hu, C.; Lutz, E.A.; Burgess, J.L. Comparison of Acute Health Effects from Exposures to Diesel and Biodiesel Fuel Emissions. J. Occup. Environ. Med. 2015, 57, 705–712. [Google Scholar] [CrossRef]
- Selley, L.; Phillips, D.H.; Mudway, I. The Potential of Omics Approaches to Elucidate Mechanisms of Biodiesel-Induced Pulmonary Toxicity. Part. Fibre Toxicol. 2019, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Jalava, P.I.; Tapanainen, M.; Kuuspalo, K.; Markkanen, A.; Hakulinen, P.; Happo, M.S.; Pennanen, A.S.; Ihalainen, M.; Yli-Pirilä, P.; Makkonen, U.; et al. Toxicological Effects of Emission Particles from Fossil- and Biodiesel-Fueled Diesel Engine with and without DOC/POC Catalytic Converter. Inhal. Toxicol. 2010, 22, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Sadiktsis, I.; Koegler, J.H.; Benham, T.; Bergvall, C.; Westerholm, R. Particulate Associated Polycyclic Aromatic Hydrocarbon Exhaust Emissions from a Portable Power Generator Fueled with Three Different Fuels–A Comparison between Petroleum Diesel and Two Biodiesels. Fuel 2014, 115, 573–580. [Google Scholar] [CrossRef]
- Godri Pollitt, K.J.; Chhan, D.; Rais, K.; Pan, K.; Wallace, J.S. Biodiesel Fuels: A Greener Diesel? A Review from a Health Perspective. Sci. Total Environ. 2019, 688, 1036–1055. [Google Scholar] [CrossRef]
- Madden, M.C. A Paler Shade of Green? The Toxicology of Biodiesel Emissions: Recent Findings from Studies with This Alternative Fuel. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 2856–2862. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Lin, T.-C.; Wang, Y.-J.; Ho, W.-L. Carbonyl Compounds and Toxicity Assessments of Emissions from a Diesel Engine Running on Biodiesels. J. Air Waste Manag. Assoc. 2009, 59, 163–171. [Google Scholar] [CrossRef]
- Feyzi, M.; Hassankhani, A.; Rafiee, H.R. Preparation and Characterization of Cs/Al/Fe3O4 Nanocatalysts for Biodiesel Production. Energy Convers. Manag. 2013, 71, 62–68. [Google Scholar] [CrossRef]
- Syazwani, O.N.; Rashid, U.; Mastuli, M.S.; Taufiq-Yap, Y.H. Esterification of Palm Fatty Acid Distillate (PFAD) to Biodiesel Using Bi-Functional Catalyst Synthesized from Waste Angel Wing Shell (Cyrtopleura costata). Renew. Energy 2019, 131, 187–196. [Google Scholar] [CrossRef]
- Kawashima, A.; Matsubara, K.; Honda, K. Development of Heterogeneous Base Catalysts for Biodiesel Production. Bioresour. Technol. 2008, 99, 3439–3443. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Pang, Y.L. Investigation of Carbon-Based Solid Acid Catalyst from Jatropha Curcas Biomass in Biodiesel Production. Energy Convers. Manag. 2017, 144, 10–17. [Google Scholar] [CrossRef]
- Mo, X.; Lotero, E.; Lu, C.; Liu, Y.; Goodwin, J.G. A Novel Sulfonated Carbon Composite Solid Acid Catalyst for Biodiesel Synthesis. Catal. Lett. 2008, 123, 1–6. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S.; Piao, X. Transesterification of Soybean Oil to Biodiesel Using CaO as a Solid Base Catalyst. Fuel 2008, 87, 216–221. [Google Scholar] [CrossRef]
- Chin, L.H.; Abdullah, A.Z.; Hameed, B.H. Sugar Cane Bagasse as Solid Catalyst for Synthesis of Methyl Esters from Palm Fatty Acid Distillate. Chem. Eng. J. 2012, 183, 104–107. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Taufiq-Yap, Y.H. Meso- and Macroporous Sulfonated Starch Solid Acid Catalyst for Esterification of Palm Fatty Acid Distillate. Arab. J. Chem. 2016, 9, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Lokman, I.M.; Rashid, U.; Taufiq-Yap, Y.H.; Yunus, R. Methyl Ester Production from Palm Fatty Acid Distillate Using Sulfonated Glucose-Derived Acid Catalyst. Renew. Energy 2015, 81, 347–354. [Google Scholar] [CrossRef]
- Shuit, S.H.; Tan, S.H. Biodiesel Production via Esterification of Palm Fatty Acid Distillate Using Sulphonated Multi-Walled Carbon Nanotubes as a Solid Acid Catalyst: Process Study, Catalyst Reusability and Kinetic Study. Bioenergy Res. 2015, 8, 605–617. [Google Scholar] [CrossRef]
- Theam, K.L.; Islam, A.; Lee, H.V.; Taufiq-Yap, Y.H. Sucrose-Derived Catalytic Biodiesel Synthesis from Low Cost Palm Fatty Acid Distillate. Process Saf. Environ. Prot. 2015, 95, 126–135. [Google Scholar] [CrossRef]
- Thitsartarn, W.; Kawi, S. Transesterification of Oil by Sulfated Zr-Supported Mesoporous Silica. Ind. Eng. Chem. Res. 2011, 50, 7857–7865. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Sulfated Tin Oxide as Solid Superacid Catalyst for Transesterification of Waste Cooking Oil: An Optimization Study. Appl. Catal. B: Environ. 2009, 93, 134–139. [Google Scholar] [CrossRef]
- Kaur, K.; Wanchoo, R.K.; Toor, A.P. Sulfated Iron Oxide: A Proficient Catalyst for Esterification of Butanoic Acid with Glycerol. Ind. Eng. Chem. Res. 2015, 54, 3285–3292. [Google Scholar] [CrossRef]
- Huang, C.-C.; Yang, C.-J.; Gao, P.-J.; Wang, N.-C.; Chen, C.-L.; Chang, J.-S. Characterization of an Alkaline Earth Metal-Doped Solid Superacid and Its Activity for the Esterification of Oleic Acid with Methanol. Green Chem. 2015, 17, 3609–3620. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Taufiq-Yap, Y.H. Production of Biodiesel from Palm Fatty Acid Distillate Using Sulfonated-Glucose Solid Acid Catalyst: Characterization and Optimization. Chin. J. Chem. Eng. 2015, 23, 1857–1864. [Google Scholar] [CrossRef]
- Thushari, I.; Babel, S. Sustainable Utilization of Waste Palm Oil and Sulfonated Carbon Catalyst Derived from Coconut Meal Residue for Biodiesel Production. Bioresour. Technol. 2018, 248, 199–203. [Google Scholar] [CrossRef]
- Chen, W.; Feng, H.; Shen, D.; Jia, Y.; Li, N.; Ying, X.; Chen, T.; Zhou, Y.; Guo, J.; Zhou, M. Carbon Materials Derived from Waste Tires as High-Performance Anodes in Microbial Fuel Cells. Sci. Total Environ. 2018, 618, 804–809. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic Pyrolysis of Plastic Waste: A Review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- De Sousa, F.D.B.; Scuracchio, C.H.; Hu, G.-H.; Hoppe, S. Devulcanization of Waste Tire Rubber by Microwaves. Polym. Degrad. Stab. 2017, 138, 169–181. [Google Scholar] [CrossRef]
- Climate Policy Watcher. Available online: https://www.climate-policy-watcher.org/scrap-tires/executive-summary.html (accessed on 29 October 2021).
- Shah, S.A.Y.; Zeeshan, M.; Farooq, M.Z.; Ahmed, N.; Iqbal, N. Co-Pyrolysis of Cotton Stalk and Waste Tire with a Focus on Liquid Yield Quantity and Quality. Renew. Energy 2019, 130, 238–244. [Google Scholar] [CrossRef]
- Labaki, M.; Jeguirim, M. Thermochemical Conversion of Waste Tyres—A Review. Environ. Sci. Pollut. Res. 2017, 24, 9962–9992. [Google Scholar] [CrossRef]
- Zeaiter, J.; Azizi, F.; Lameh, M.; Milani, D.; Ismail, H.Y.; Abbas, A. Waste Tire Pyrolysis Using Thermal Solar Energy: An Integrated Approach. Renew. Energy 2018, 123, 44–51. [Google Scholar] [CrossRef]
- Wang, W.-C.; Bai, C.-J.; Lin, C.-T.; Prakash, S. Alternative Fuel Produced from Thermal Pyrolysis of Waste Tires and Its Use in a DI Diesel Engine. Appl. Therm. Eng. 2016, 93, 330–338. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Li, J.; Lin, F.; Ma, W.; Yan, B.; Chen, G. Transformation of Nitrogen, Sulfur and Chlorine during Waste Tire Pyrolysis. J. Anal. Appl. Pyrolysis 2021, 153, 104987. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Jiang, L.; Zhang, W.; Li, R.; Chi, Y. HCl and PCDD/Fs Emission Characteristics from Incineration of Source-Classified Combustible Solid Waste in Fluidized Bed. RSC Adv. 2015, 5, 67866–67873. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray Diffraction Patterns of Graphite and Turbostratic Carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Ngaosuwan, K.; Goodwin, J.G.; Prasertdham, P. A Green Sulfonated Carbon-Based Catalyst Derived from Coffee Residue for Esterification. Renew. Energy 2016, 86, 262–269. [Google Scholar] [CrossRef]
- Liu, T.; Li, Z.; Li, W.; Shi, C.; Wang, Y. Preparation and Characterization of Biomass Carbon-Based Solid Acid Catalyst for the Esterification of Oleic Acid with Methanol. Bioresour. Technol. 2013, 133, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Bureros, G.M.A.; Tanjay, A.A.; Cuizon, D.E.S.; Go, A.W.; Cabatingan, L.K.; Agapay, R.C.; Ju, Y.-H. Cacao Shell-Derived Solid Acid Catalyst for Esterification of Oleic Acid with Methanol. Renew. Energy 2019, 138, 489–501. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, A.; Meng, Y.; Wang, L.; Jiang, H.; Li, G. Catalytic Performance of Biomass Carbon-Based Solid Acid Catalyst for Esterification of Free Fatty Acids in Waste Cooking Oil. Catal. Surv. Asia 2015, 19, 61–67. [Google Scholar] [CrossRef]
- Da Luz Corrêa, A.P.; Bastos, R.R.C.; da Rocha Filho, G.N.; Zamian, J.R.; da Conceição, L.R.V. Preparation of Sulfonated Carbon-Based Catalysts from Murumuru Kernel Shell and Their Performance in the Esterification Reaction. RSC Adv. 2020, 10, 20245–20256. [Google Scholar] [CrossRef]
- Tang, X.; Niu, S. Preparation of Carbon-Based Solid Acid with Large Surface Area to Catalyze Esterification for Biodiesel Production. J. Ind. Eng. Chem. 2019, 69, 187–195. [Google Scholar] [CrossRef]
- Deyab, M.A.; Corrêa, R.G.C.; Mazzetto, S.E.; Dhmees, A.S.; Mele, G. Improving the Sustainability of Biodiesel by Controlling the Corrosive Effects of Soybean Biodiesel on Aluminum Alloy 5052 H32 via Cardanol. Ind. Crops Prod. 2019, 130, 146–150. [Google Scholar] [CrossRef]
- Roeges, N.P.; Baas, J.M. A Guide to the Complete Interpretation of Infrared Spectral of Organic Structures; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 10815–10837. [Google Scholar]
- Siatis, N.G.; Kimbaris, A.C.; Pappas, C.S.; Tarantilis, P.A.; Polissiou, M.G. Improvement of Biodiesel Production Based on the Application of Ultrasound: Monitoring of the Procedure by FTIR Spectroscopy. J. Am. Oil Chem. Soc. 2006, 83, 53–57. [Google Scholar] [CrossRef]













| Waste Raw Material | Sulfonating Agent | Reaction Conditions | Conversion Efficiency (%) | Ref. | |||
|---|---|---|---|---|---|---|---|
| Temp. (°C) | Time (Minutes) | Molar Ratio | Catalyst (wt.%) | ||||
| Coffee Residue | Concentrated H2SO4 | 60 | 240 | 3:1 | 5 | 71.5 | [56] |
| Sugar Cane Bagasse | Concentrated H2SO4 | 170 | 30 | 20:1 | 11.5 | 80 | [34] |
| Coconut Meal Residue | Concentrated H2SO4 | 65–70 | 720 | 12:1 | 5 | 92.7 | [44] |
| Corn Straw | Concentrated H2SO4 | 60 | 240 | 7:1 | 7 | 93 | [57] |
| Cacao Shell | Concentrated H2SO4 | 42 | 1440 | 7:1 | 5 | 93 | [58] |
| Sugar Cane Bagasse | Concentrated H2SO4 | 66 | 240 | 18:1 | 1 | 94.4 | [59] |
| D-glucose | Concentrated H2SO4 | 65 | 134 | 12.2:1 | 2.9 | 94.5 | [43] |
| Murumuru Kernel Shells | Concentrated H2SO4 | 90 | 90 | 10:1 | 5 | 97.2 | [60] |
| Bamboo | Concentrated H2SO4 | 65 | 120 | 10:1 | 10 | 97.3 | [61] |
| Tire Polymer Waste | Concentrated H2SO4 | 70 | 120 | 15:1 | 5 | 98.8 | Present Study |
| S. No. | Parameters | Unit | FAME | EN 14214 |
|---|---|---|---|---|
| 1 | Flash Point | °C | 152 | 100–170 |
| 2 | Cloud Point | °C | 0 | - |
| 3 | Pour Point | °C | −2 | −5–10 |
| 4 | Kinematic Viscosity (40 °C) | cSt | 4.68 | 1.9–6.0 |
| 5 | Density (31 °C) | Kg mm−3 | 881 | 860–900 |
| 6 | Acid Value | mg KOH g−1 | 0.5 | 0.8 |
| 7 | Ash Content | wt.% | Nil | 0.02 |
| 8 | Iodine Index | g I2 100 g−1 | 120 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maafa, I.M. Biodiesel Synthesis from High Free-Fatty-Acid Chicken Fat using a Scrap-Tire Derived Solid Acid Catalyst and KOH. Polymers 2022, 14, 643. https://doi.org/10.3390/polym14030643
Maafa IM. Biodiesel Synthesis from High Free-Fatty-Acid Chicken Fat using a Scrap-Tire Derived Solid Acid Catalyst and KOH. Polymers. 2022; 14(3):643. https://doi.org/10.3390/polym14030643
Chicago/Turabian StyleMaafa, Ibrahim M. 2022. "Biodiesel Synthesis from High Free-Fatty-Acid Chicken Fat using a Scrap-Tire Derived Solid Acid Catalyst and KOH" Polymers 14, no. 3: 643. https://doi.org/10.3390/polym14030643
APA StyleMaafa, I. M. (2022). Biodiesel Synthesis from High Free-Fatty-Acid Chicken Fat using a Scrap-Tire Derived Solid Acid Catalyst and KOH. Polymers, 14(3), 643. https://doi.org/10.3390/polym14030643